��Ŀ����
A��B��C��D��E��F��ԭ���������������ǰ������Ԫ�أ�A��B��C����Ԫ�صĻ�̬ԭ�Ӿ�����ͬ���ܼ����ܲ㣬�ҵ�һ�����ܵ�˳��ΪA��C��B������Cԭ�ӻ�̬ʱ2p���������δ�ɶԵ��ӣ�D��Aͬ�壬�䵥��Ϊ���õİ뵼����ϣ�EΪǰ�����ڵ縺����С��Ԫ�أ�Fԭ���ڲ��չ����������������E��ͬ����ش�
��1��F+�ļ۲�����Ų�Ϊ ��д��������F2C��ϡ���ᷴӦ�IJ���֮һΪ��ɫ�����ҷ�Ӧ��ֻ��FԪ�ػ��ϼ۷����˱仯���÷�Ӧ�����ӷ���ʽ ��
��2��B��D�γ�һ�ֳ�Ӳ����ĥ�����µ����ͻ�����û����ﻯѧʽΪ ������ ���壮
��������C2��Ӧ����1mol��̬H2C�������241.8KJ����1g ��̬H2Cת����Һ̬H2C����2.444KJ����Ӧ��2H2��g��+C2��g��=2H2C��L���ġ�H= KJ/mol��
��3��C����Ԫ���γɵ���һ�����Ӻ���10���ӣ���������Cԭ�ӵ��ӻ���ʽΪ �� C����Ԫ���γɵ�һ��18���ӷ���M��ˮ��Һ���������ԣ���M��Ba��OH��2��Һ��Ӧ������ʽ�εĻ�ѧ����ʽΪ ��
��4��1molAC2�����ЦҼ���м�����Ŀ֮��Ϊ ��B��C�γɵ���һ������N��AC2��Ϊ�ȵ����壬N�ĵ���ʽΪ �������£���1molAC2������ʵ�����NaOH��Ӧ�����ҺpH����9����c�� H2CO3��-c�� CO32-��= ��
��1��F+�ļ۲�����Ų�Ϊ
��2��B��D�γ�һ�ֳ�Ӳ����ĥ�����µ����ͻ�����û����ﻯѧʽΪ
��������C2��Ӧ����1mol��̬H2C�������241.8KJ����1g ��̬H2Cת����Һ̬H2C����2.444KJ����Ӧ��2H2��g��+C2��g��=2H2C��L���ġ�H=
��3��C����Ԫ���γɵ���һ�����Ӻ���10���ӣ���������Cԭ�ӵ��ӻ���ʽΪ
��4��1molAC2�����ЦҼ���м�����Ŀ֮��Ϊ
������A��B��C��D��E��F��ԭ���������������ǰ������Ԫ�أ�EΪǰ�����ڵ縺����С��Ԫ�أ���EΪKԪ�أ�A��B��C����Ԫ�صĻ�̬ԭ�Ӿ�����ͬ���ܼ����ܲ㣬���ִ���ͬһ���ڣ�����Cԭ�ӻ�̬ʱ2p���������δ�ɶԵ��ӣ�����Ԫ�ش��ڵڶ����ڣ�Cԭ����Χ�����Ų�Ϊ2s22p2��2s22p4����һ�����ܵ�˳��ΪA��C��B����BԪ��ԭ�ӵ�2p�ܼ�Ϊ�����ȶ�״̬������Χ�����Ų�Ϊ2s22p3����BΪ��Ԫ�أ�Cԭ����Χ�����Ų�Ϊ2s22p4����CΪ��Ԫ�أ�Aԭ����Χ�����Ų�Ϊ2s22p1��2s22p2��D��Aͬ�壬�䵥��Ϊ���õİ뵼����ϣ����ڢ�A�壬��AΪ̼Ԫ�ء�DΪSiԪ�أ�Fԭ���ڲ��չ����������������E��ͬ����Fԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s1����FΪCuԪ�أ��ݴ˽��
����⣺A��B��C��D��E��F��ԭ���������������ǰ������Ԫ�أ�EΪǰ�����ڵ縺����С��Ԫ�أ���EΪKԪ�أ�A��B��C����Ԫ�صĻ�̬ԭ�Ӿ�����ͬ���ܼ����ܲ㣬���ִ���ͬһ���ڣ�����Cԭ�ӻ�̬ʱ2p���������δ�ɶԵ��ӣ�����Ԫ�ش��ڵڶ����ڣ�Cԭ����Χ�����Ų�Ϊ2s22p2��2s22p4����һ�����ܵ�˳��ΪA��C��B����BԪ��ԭ�ӵ�2p�ܼ�Ϊ�����ȶ�״̬������Χ�����Ų�Ϊ2s22p3����BΪ��Ԫ�أ�Cԭ����Χ�����Ų�Ϊ2s22p4����CΪ��Ԫ�أ�Aԭ����Χ�����Ų�Ϊ2s22p1��2s22p2��D��Aͬ�壬�䵥��Ϊ���õİ뵼����ϣ����ڢ�A�壬��AΪ̼Ԫ�ء�DΪSiԪ�أ�Fԭ���ڲ��չ����������������E��ͬ����Fԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s1����FΪCuԪ�أ�
��1��FΪCuԪ�أ��Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s1��Cu+�ļ۲�����Ų�Ϊ3d10��
������Cu2O��ϡ���ᷴӦ�IJ���֮һΪ��ɫ�����ҷ�Ӧ��ֻ��CuԪ�ػ��ϼ۷����˱仯��Ӧ����Cu������ͭ��ͬʱ����ˮ���÷�Ӧ�����ӷ���ʽCu2O+2H+=Cu2++Cu+H2O��
�ʴ�Ϊ��3d10��Cu2O+2H+=Cu2++Cu+H2O��
��2��N��Si�γ�һ�ֳ�Ӳ����ĥ�����µ����ͻ�����û����ﻯѧʽΪSi3N4������ԭ�Ӿ��壬��������O2��Ӧ����1mol��̬H2O�������241.8KJ����1g��̬H2Oת����Һ̬H2O����2.444KJ��1mol��̬H2Oת����Һ̬H2O����Ϊ2.444KJ��
=44kJ��������2molҺ̬H2O�ų�������Ϊ��241.8kJ+44kJ����
=571.6kJ����Ӧ��2H2��g��+O2��g��=2H2O��l���ġ�H=-571.6kJ/mol��
�ʴ�Ϊ��Si3N4��ԭ�ӣ�-571.6��
��3����Ԫ������Ԫ���γɵ���һ�����Ӻ���10����ΪH3O+��H3O+����ԭ�Ӽ۲���Ӷ���=3+
=4��Oԭ�Ӻ���1�Թ¶Ե��ӣ���Oԭ�Ӳ���sp3�ӻ���
��Ԫ������Ԫ���γɵ�һ��18���ӷ���M��ˮ��Һ���������ԣ�MΪH2O2����H2O2��Ba��OH��2��Һ��Ӧ������ʽ�εĻ�ѧ����ʽΪ��2H2O2+Ba��OH��2=Ba��HO2��2+2H2O��
�ʴ�Ϊ��sp3��2H2O2+Ba��OH��2=Ba��HO2��2+2H2O��
��4��CO2���ӽṹʽΪO=C=O��1molCO2�����к���2mol�Ҽ���2mol�м����ʺ��ЦҼ���м�����Ŀ֮��Ϊ1��1��N��O��Ԫ���γɵ���һ������N��CO2��Ϊ�ȵ����壬��NΪNO2+���ṹ��CO2���ƣ�NO2+�ĵ���ʽΪ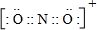 ��
��
�����£���1molCO2������ʵ�����NaOH��Ӧ��������ҺΪNaHCO3��Һ����ҺpH����9�����ݵ���غ���c��OH-��+2c�� CO32-��+c��HCO3-��=c��H+��+c��Na+�������������غ���c��Na+��=c�� CO32-��+c��HCO3-��+c�� H2CO3���������ɵ�c�� H2CO3��-c�� CO32-��=c��OH-��-c��H+��=
mol/L-10-9mol/L=��10-5-10-9��mol/L��
�ʴ�Ϊ��1��1��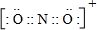 ����10-5-10-9��mol/L��
����10-5-10-9��mol/L��
��1��FΪCuԪ�أ��Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s1��Cu+�ļ۲�����Ų�Ϊ3d10��
������Cu2O��ϡ���ᷴӦ�IJ���֮һΪ��ɫ�����ҷ�Ӧ��ֻ��CuԪ�ػ��ϼ۷����˱仯��Ӧ����Cu������ͭ��ͬʱ����ˮ���÷�Ӧ�����ӷ���ʽCu2O+2H+=Cu2++Cu+H2O��
�ʴ�Ϊ��3d10��Cu2O+2H+=Cu2++Cu+H2O��
��2��N��Si�γ�һ�ֳ�Ӳ����ĥ�����µ����ͻ�����û����ﻯѧʽΪSi3N4������ԭ�Ӿ��壬��������O2��Ӧ����1mol��̬H2O�������241.8KJ����1g��̬H2Oת����Һ̬H2O����2.444KJ��1mol��̬H2Oת����Һ̬H2O����Ϊ2.444KJ��
| 1mol��18g/mol |
| 1g |
| 2mol |
| 1mol |
�ʴ�Ϊ��Si3N4��ԭ�ӣ�-571.6��
��3����Ԫ������Ԫ���γɵ���һ�����Ӻ���10����ΪH3O+��H3O+����ԭ�Ӽ۲���Ӷ���=3+
| 6-1��3-1 |
| 2 |
��Ԫ������Ԫ���γɵ�һ��18���ӷ���M��ˮ��Һ���������ԣ�MΪH2O2����H2O2��Ba��OH��2��Һ��Ӧ������ʽ�εĻ�ѧ����ʽΪ��2H2O2+Ba��OH��2=Ba��HO2��2+2H2O��
�ʴ�Ϊ��sp3��2H2O2+Ba��OH��2=Ba��HO2��2+2H2O��
��4��CO2���ӽṹʽΪO=C=O��1molCO2�����к���2mol�Ҽ���2mol�м����ʺ��ЦҼ���м�����Ŀ֮��Ϊ1��1��N��O��Ԫ���γɵ���һ������N��CO2��Ϊ�ȵ����壬��NΪNO2+���ṹ��CO2���ƣ�NO2+�ĵ���ʽΪ
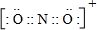 ��
�������£���1molCO2������ʵ�����NaOH��Ӧ��������ҺΪNaHCO3��Һ����ҺpH����9�����ݵ���غ���c��OH-��+2c�� CO32-��+c��HCO3-��=c��H+��+c��Na+�������������غ���c��Na+��=c�� CO32-��+c��HCO3-��+c�� H2CO3���������ɵ�c�� H2CO3��-c�� CO32-��=c��OH-��-c��H+��=
| 10-14 |
| 10-9 |
�ʴ�Ϊ��1��1��
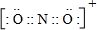 ����10-5-10-9��mol/L��
����10-5-10-9��mol/L��������������Ԫ���ƶ�Ϊ���壬�����������Ų����ɡ����û�ѧ������д����ѧ��������Ũ�ȱȽϵȣ��Ѷ��еȣ���4���е���ʽ������Ũ�ȹ�ϵΪ�״��㡢�ѵ㣬ע��ȵ������м۲��������ͬ����ṹ���ƣ�����ˮ��������Ũ�ȵ�����ϵ�����������غ㡢���غ㡢���Ӻ��ʽ��

��ϰ��ϵ�д�
�����Ŀ
 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�