��Ŀ����
����Ŀ����ҵ��NaCl��NH3��CO2��Ϊԭ�����Ƶ�NaHCO3����������������йط�Ӧ�Ļ�ѧ����ʽΪ��
NH3��CO2��H2O===NH4HCO3
NH4HCO3��NaCl===NaHCO3����NH4Cl
2NaHCO3![]() Na2CO3��CO2����H2O
Na2CO3��CO2����H2O
(1)̼������뱥��ʳ��ˮ��Ӧ��������̼�����ƾ����ԭ����__________(����ĸ���)��
a��̼������������ˮ
b��̼�����������ֽ�
c��̼�����Ƶ��ܽ����Խ�С����������Һ�����Ƚᾧ����
d��̼�����Ƶ��ȶ��Դ���̼����
(2)ij�С����������Ƽ�ԭ��������̼�����Ƶ��Ʊ�ʵ�顣
��һλͬѧ��������̼����ͨ�뺬���ı���ʳ��ˮ���Ʊ�̼�����ƣ�ʵ��װ������ͼ��ʾ(ͼ�мг֡��̶��õ�����δ����)��


�Իش������й����⣺
(��)��װ���е��Լ���____________����������____________________________��
(��)��װ����ϡ�����������_______________________��
(��)ʵ����������NaHCO3 ����IJ�����__________(��������������)���ò�������Ҫ�IJ���������__________________________��
����һλͬѧ��ͼ����װ��(����װ��δ����)����ʵ�顣
(��)ʵ��ʱ�����ȴ�a��ͨ��________���壬˵��ԭ��______________________��
(��)��ͬѧ��������װ�õ�b���¶����Ӽ�װ�ã�������____________________��
(3)̼�������������ù���12.28 g��������ʯ��ˮ��ַ�Ӧ�����ó�����ϴ�ӡ���������Ϊ12.00 g�������ù�����̼���Ƶ���������Ϊ__________________��
���𰸡�(1)c
(2)��(��)����̼��������Һ ��ȥCO2�е�HCl����
(��)����δ��Ӧ���NH3
(��)���� ��������©�����ձ�
��(��)NH3 ���������ܽ���ˮ�����γɽϴ�Ũ�ȵ���Һ�������ڶ�����̼���գ����ɸ����̼����� (��)������������Һ�Ӵ���������CO2��������
(3)86.3%(��0.863)
��������(1)̼������뱥��ʳ��ˮ��Ӧ��������̼�����ƾ����ԭ����̼�����Ƶ��ܽ����Խ�С����������Һ�����Ƚᾧ������ѡc��(2)��(��)��װ��������ȥ������̼�е��Ȼ������ʣ���ʢ�Լ��DZ���̼��������Һ��(��)ע�������Ƕ�װ����ϡ��������ã��������ʶ��е���ĩ�����ӵ���©�������ã����Դ�����δ��Ӧ���NH3��(��)ʵ�������NaHCO3 �������Һ�������������ù��˷����룻���˲����õ����ֲ�����������������©�����ձ�����(��)ʵ��ʱ�����ȴ�a��ͨ�백�������������ܽ���ˮ�����γɽϴ�Ũ�ȵ���Һ�������ڶ�����̼���գ����ɸ����̼����李�(��)����װ�õ�b���¶����Ӽ�װ�ã���������������Һ�Ӵ���������CO2�������ʡ�(3)��̼�������������ù�����̼���Ƶ����ʵ�����x mol��̼�����Ƶ����ʵ�����y mol����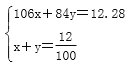 ��ã�x��0.1��y��0.02������̼���Ƶ���������Ϊ
��ã�x��0.1��y��0.02������̼���Ƶ���������Ϊ![]() ��100%��86.3%��
��100%��86.3%��