��Ŀ����
����ڿ�ѧ����������������Ҫ��Ӧ�á�ʯ��ϼ���һ�ֺܺõ�ֲ��ɱ��������������ʯ�ҡ�ˮ�ȷ�Ӧ���èD�D�ɶ���[ CaSx��x��5��]������������ɵĻ�����ͬ������ʯ�Һ������ˮ��Һ�з�Ӧ�������ɲ�ͬ�Ķ��ƺ��������ơ��磺
3Ca(OH)2+8S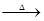 2CaS3+CaS2O3+3H2O��
2CaS3+CaS2O3+3H2O��
3Ca(OH)2+6S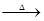 2CaS2+CaS2O3+3H2O�ȡ�
2CaS2+CaS2O3+3H2O�ȡ�
��������������м��㣺
��1��������ܽ���������Һ������һ�ֶ�����ö���������Ԫ�ص���������Ϊ0.736��ͨ������ȷ��Na2Sx��x= ��
��2����ȡijʯ��ϼ�������ֻ�����ֺ����250mL���ܶ�Ϊ1.12g/mL��ͨ������CO2ʹ����ٷֽ⡣ԭ�����£�
Sx2-+2H2O+2CO2��(x-1)S��+H2S��+ 2HCO3-��S2O32- + H2O+CO2��HSO3-+HCO3-+S�� ��
��ȫ��Ӧ���ռ�������1.12����S��P��T����������������Һ�е��ܽ⣩�����ʯ��ϼ��ж��Ƶ����ʵ���Ũ��Ϊ ���������Ƶ���������Ϊ ��������С����ʾ������2λ��Ч���֣�
��3�����������ʯ�ҡ���Ǻ�ˮ��������7��16��70�������ȫ��Ӧ��ֻ�������ֺ���Ļ�������ʯ��ϼ��ж������������Ƶ����ʵ���֮���� �����ƵĻ�ѧʽ�� ��
��4����������ʯ��50.4g�����96g��ˮ504gǡ����ȫ��Ӧ�������ֶ��ƣ�������CaS4֮��ж��ֶ��ơ�����������ֶ��ƿ��ܵ����ʵ���֮�ȣ�д��������̣���
3Ca(OH)2+8S
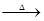 2CaS3+CaS2O3+3H2O��
2CaS3+CaS2O3+3H2O��3Ca(OH)2+6S
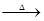 2CaS2+CaS2O3+3H2O�ȡ�
2CaS2+CaS2O3+3H2O�ȡ���������������м��㣺
��1��������ܽ���������Һ������һ�ֶ�����ö���������Ԫ�ص���������Ϊ0.736��ͨ������ȷ��Na2Sx��x= ��
��2����ȡijʯ��ϼ�������ֻ�����ֺ����250mL���ܶ�Ϊ1.12g/mL��ͨ������CO2ʹ����ٷֽ⡣ԭ�����£�
Sx2-+2H2O+2CO2��(x-1)S��+H2S��+ 2HCO3-��S2O32- + H2O+CO2��HSO3-+HCO3-+S�� ��
��ȫ��Ӧ���ռ�������1.12����S��P��T����������������Һ�е��ܽ⣩�����ʯ��ϼ��ж��Ƶ����ʵ���Ũ��Ϊ ���������Ƶ���������Ϊ ��������С����ʾ������2λ��Ч���֣�
��3�����������ʯ�ҡ���Ǻ�ˮ��������7��16��70�������ȫ��Ӧ��ֻ�������ֺ���Ļ�������ʯ��ϼ��ж������������Ƶ����ʵ���֮���� �����ƵĻ�ѧʽ�� ��
��4����������ʯ��50.4g�����96g��ˮ504gǡ����ȫ��Ӧ�������ֶ��ƣ�������CaS4֮��ж��ֶ��ơ�����������ֶ��ƿ��ܵ����ʵ���֮�ȣ�д��������̣���
��1����4 ��2�֣� ��2����0.20 mol/L ��2�֣� 0.014 ��2�֣�
��3����2:1 ��2�֣� CaS5 ��2�֣�
��4����n(CaO)="50.4/56" =0.3mol n(S)="96/32" =3mol ��1�֣�
�� n[Ca(OH)2 ]: n(S) =" 3" : 10 �ɷ�Ӧ��֪��3Ca(OH)2+10S 2CaSx+CaS2O3+3H2O
2CaSx+CaS2O3+3H2O
��֮�ã�x="4" ������������ԭ�ӵ�ƽ��ֵΪ4�� ��1�֣�
�������ֶ��������Ϊ��CaS2��CaS5��CaS3��CaS5�� ��2�֣�
��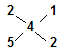 ��֪��n(CaS2)��n(CaS5)=1:2����1�֣�
��֪��n(CaS2)��n(CaS5)=1:2����1�֣�
��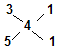 ��֪��n(CaS3)��n(CaS5)=1:1 ��1�֣�
��֪��n(CaS3)��n(CaS5)=1:1 ��1�֣�
���������ⷨҲ���Ը��֡�
��3����2:1 ��2�֣� CaS5 ��2�֣�
��4����n(CaO)="50.4/56" =0.3mol n(S)="96/32" =3mol ��1�֣�
�� n[Ca(OH)2 ]: n(S) =" 3" : 10 �ɷ�Ӧ��֪��3Ca(OH)2+10S
 2CaSx+CaS2O3+3H2O
2CaSx+CaS2O3+3H2O��֮�ã�x="4" ������������ԭ�ӵ�ƽ��ֵΪ4�� ��1�֣�
�������ֶ��������Ϊ��CaS2��CaS5��CaS3��CaS5�� ��2�֣�
��
 ��֪��n(CaS2)��n(CaS5)=1:2����1�֣�
��֪��n(CaS2)��n(CaS5)=1:2����1�֣���
 ��֪��n(CaS3)��n(CaS5)=1:1 ��1�֣�
��֪��n(CaS3)��n(CaS5)=1:1 ��1�֣����������ⷨҲ���Ը��֡�
��1��. ��Ԫ�ص���������Ϊ0.736������Ԫ�ص���������Ϊ0.264��������ԭ�Ӻ���ԭ�ӵĸ���֮����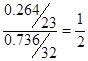 ������x��4.
������x��4.
��2��������0.05mol�����Ը��ݷ���ʽ��֪�����Ƶ����ʵ�����0.05mol����Ũ����0.05mol��0.25L��0.20 mol/L������+CaS2O3�����ʵ�����0.05mol��2��0.025mol������������������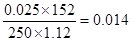 ��
��
��3����ʯ�ҡ���ǵ�������7��16�������ʵ���֮����1�U4�����������ƺ���ǵ����ʵ���֮����1�U4�����Է���ʽΪ3Ca(OH)2+12S 2CaSx+CaS2O3+3H2O�����x��5��
2CaSx+CaS2O3+3H2O�����x��5��
��4��n(CaO)="50.4/56" =0.3mol n(S)="96/32" ="3mol"
�� n[Ca(OH)2 ]: n(S) =" 3" : 10 �ɷ�Ӧ��֪��3Ca(OH)2+10S 2CaSx+CaS2O3+3H2O
2CaSx+CaS2O3+3H2O
��֮�ã�x="4" ������������ԭ�ӵ�ƽ��ֵΪ4��
�������ֶ��������Ϊ��CaS2��CaS5��CaS3��CaS5��
�� ��֪��n(CaS2)��n(CaS5)=1:2��
��֪��n(CaS2)��n(CaS5)=1:2��
�� ��֪��n(CaS3)��n(CaS5)=1:1
��֪��n(CaS3)��n(CaS5)=1:1
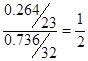 ������x��4.
������x��4.��2��������0.05mol�����Ը��ݷ���ʽ��֪�����Ƶ����ʵ�����0.05mol����Ũ����0.05mol��0.25L��0.20 mol/L������+CaS2O3�����ʵ�����0.05mol��2��0.025mol������������������
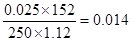 ��
����3����ʯ�ҡ���ǵ�������7��16�������ʵ���֮����1�U4�����������ƺ���ǵ����ʵ���֮����1�U4�����Է���ʽΪ3Ca(OH)2+12S
 2CaSx+CaS2O3+3H2O�����x��5��
2CaSx+CaS2O3+3H2O�����x��5����4��n(CaO)="50.4/56" =0.3mol n(S)="96/32" ="3mol"
�� n[Ca(OH)2 ]: n(S) =" 3" : 10 �ɷ�Ӧ��֪��3Ca(OH)2+10S
 2CaSx+CaS2O3+3H2O
2CaSx+CaS2O3+3H2O��֮�ã�x="4" ������������ԭ�ӵ�ƽ��ֵΪ4��
�������ֶ��������Ϊ��CaS2��CaS5��CaS3��CaS5��
��
 ��֪��n(CaS2)��n(CaS5)=1:2��
��֪��n(CaS2)��n(CaS5)=1:2����
 ��֪��n(CaS3)��n(CaS5)=1:1
��֪��n(CaS3)��n(CaS5)=1:1 
��ϰ��ϵ�д�
�����Ŀ