��Ŀ����
6����֪��ϩ�ܷ�������ת����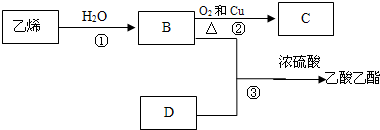
��1��д����Ӧ�Ļ�ѧ����ʽ
�٣�CH2=CH2+H2O$\stackrel{����}{��}$ CH3CH2OH ��Ӧ���ͣ��ӳɷ�Ӧ
�ڣ�2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O ����Ӧ���ͣ�������Ӧ
�ۣ�CH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O��Ӧ���ͣ�������Ӧ����ȡ����Ӧ��
��2����ϩ����ϩ�Ĺ�ϵ�ǻ�Ϊͬϵ���ϩ�����Ӿ۷�Ӧ�ķ���ʽ��nCH2=CHCH3$\stackrel{һ������}{��}$
 ��
��
���� ��ϩ��ˮ�����ӳɷ�Ӧ����BΪCH3CH2OH���Ҵ���Cu�����������·���������Ӧ����CΪCH3CHO��B��D��Ӧ��������������CH3COOCH2CH3������DΪCH3COOH���ݴ˽��
��� �⣺��ϩ��ˮ�����ӳɷ�Ӧ����BΪCH3CH2OH���Ҵ���Cu�����������·���������Ӧ����CΪCH3CHO��B��D��Ӧ��������������CH3COOCH2CH3������DΪCH3COOH��
��1����Ӧ�ٵĻ�ѧ��Ӧ����ʽΪ��CH2=CH2+H2O$\stackrel{����}{��}$ CH3CH2OH�����ڼӳɷ�Ӧ��
��Ӧ�ڵĻ�ѧ��Ӧ����ʽΪ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O������������Ӧ��
��Ӧ�۵Ļ�ѧ��Ӧ����ʽΪ��CH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O������������Ӧ����ȡ����Ӧ���ʴ�Ϊ��CH2=CH2+H2O$\stackrel{����}{��}$ CH3CH2OH���ӳɷ�Ӧ��
2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��������Ӧ��
CH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O��������Ӧ����ȡ����Ӧ����
��2����ϩ����ϩ��Ϊͬϵ���ϩ�����Ӿ۷�Ӧ�ķ���ʽ�ǣ�nCH2=CHCH3$\stackrel{һ������}{��}$ ��
��
�ʴ�Ϊ����Ϊͬϵ�nCH2=CHCH3$\stackrel{һ������}{��}$ ��
��
���� ���⿼���л����ƶϣ��漰ϩ�봼��ȩ������֮���ת�����ѶȲ���ע�����֪ʶ���������գ�

| A�� | pH=13��NaOH��Һ�к���H+��Ũ��Ϊ0��l mol/L | |
| B�� | ��NaHCO3��Һ�м�������NaOH���壬������HCO3-��ˮ�⣬ʹc��HCO3-������ | |
| C�� | ����������CH3COOH��Һ��NaOH��Һ��ϣ�������Һ�����ڣ� C��Na+��+c��H+��=c��CH3COO-��+c��OH-�� | |
| D�� | Na2CO3��Һ�У�c��Na+����c��CO32-����c��OH-��=c��HCO3-����c��H+�� |
| A�� | ԭ����ǻ�ѧ��ת��Ϊ���ܵ�װ�� | |
| B�� | ����ԭ��ص������������������ֲ�ͬ�Ľ��� | |
| C�� | ԭ��ع���ʱ����Һ�е����������ƶ� | |
| D�� | ԭ��طŵ�ʱ�������ɸ�������Һ������ |
| A�� | NaCl���Ȼ��ƾ���ķ���ʽ | |
| B�� | �Ȼ��ƾ�����һ������������һ�������� | |
| C�� | NaCl�����в����ڵ������� | |
| D�� | Na+��Cl-�Ļ�̬���������Ų�����3s23p6 |
| A�� | ������ | B�� | ��ƿ | C�� | ©�� | D�� | ��Һ©�� |

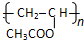 ��
��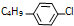 ����R��4�֣�
����R��4�֣�
 �������ӻ��������ӡ����ۡ�����
�������ӻ��������ӡ����ۡ�����