��Ŀ����
16�� ������Ԫ��A��B��C��D�����ڱ��е�λ����ͼ��E2+��D�ļ�����������ͬ�ĵ��Ӳ�ṹ���ش��������⣺
������Ԫ��A��B��C��D�����ڱ��е�λ����ͼ��E2+��D�ļ�����������ͬ�ĵ��Ӳ�ṹ���ش��������⣺��1����A��B��C��D����Ԫ�ص�ԭ������֮��Ϊm����mB��
A��һ��Ϊ���� B��һ��Ϊż�� C������Ϊ������Ҳ����Ϊż��
��2��DԪ��ԭ�ӵĴ������������������������֮�ͣ���
��д��A�γɵļ����ӵĽṹʾ��ͼ
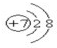 ��Ԫ��Dλ��Ԫ�����ڱ��ĵڢ�A�壮
��Ԫ��Dλ��Ԫ�����ڱ��ĵڢ�A�壮��AԪ�ص�һ���⻯���������6��ԭ�ӣ���ṹ��ʽΪH2N-NH2����ѹ298Kʱ0.2mol����̬�⻯����O2 ����ȫȼ�գ�������̬A���ʺ�ˮ���ų�����106.8kJ������̬�⻯��ȼ���ȵ��Ȼ�ѧ����ʽΪN2H4��g��+O2��g��=N2��g��+2H2O��l����H=-534kJ/mol��
��д��C������B������⻯�ﷴӦ�Ļ�ѧ����ʽ��2H2O+2F2=4HF+O2��
���õ���ʽ��ʽEB2���γɹ��̣�
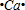 +2
+2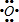 ��
��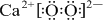 ��
����ʵ��֤ʵAC3��ˮ�ᷢ����Ӧ����AC3��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��NF3+4NaOH=NaNO2+3NaF+2H2O��
���� ��1�����B��ԭ����������������Ԫ�ص����λ�õó�����Ԫ�ص�ԭ������֮�ͣ�Ȼ���ж�m��
��2������DԪ��ԭ�ӵĴ������������������������֮�Ϳ�֪DΪS����BΪO��AΪN��CΪFԪ�أ�
��Nԭ���γɵļ�������N3-����˵����Ϊ7�������������Ϊ10���ݴ�д�������ӽṹʾ��ͼ��DΪS��λ�����ڱ��е������ڢ�A�壻
��AΪN��6��ԭ�ӵ��⻯��ΪN2H4����ṹ��ʽΪH2N-NH2�������1molN2H4��ȫȼ�շų�������Ȼ��д���÷�Ӧ��ȼ���ȵ��Ȼ�ѧ����ʽ��
��C����ΪF2��B���⻯��ΪH2O��д��������ˮ��Ӧ�Ļ�ѧ����ʽ��
��E2+��D�ļ�����������ͬ�ĵ��Ӳ�ṹ����EΪCaԪ�أ�EB2ΪCaO2���������ӻ�����õ���ʽ��ʾ�����γɹ��̣�
�ݸ�����ϢNF3��ˮ��ӦΪ��NF3+2H2O=HNO2+3HF�������ᡢ���������������Ʒ�Ӧ���ɷ����ơ��������ƺ�ˮ���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��� �⣺��1������A��B��C��D����Ԫ�������ڱ������λ�ÿ�֪��Dֻ��λ�����ڱ��������ڣ���B��ԭ������Ϊx����ԭ������A��x-1��C��x+1��D��x+8��A��B��C��D����Ԫ�ص�ԭ������֮��Ϊ����x-1��+x+��x+1��+��x+8��=4x+8����m��Ϊż������B��ȷ��
�ʴ�Ϊ��B��
��2������DԪ��ԭ�ӵĴ������������������������֮�Ϳ�֪DΪS����BΪO��AΪN��CΪFԪ�أ�
��AΪNԪ�أ�Nԭ���γɵļ�������N3-����˵����Ϊ7�������ﵽ8�����ȶ��ṹ�������ӽṹʾ��ͼΪ�� ��DΪSԪ�أ�λ�����ڱ���VIA�壬
��DΪSԪ�أ�λ�����ڱ���VIA�壬
�ʴ�Ϊ�� ��VIA��
��VIA��
��AΪN��6��ԭ�ӵ��⻯��ΪN2H4��Ϊ���ۻ������ṹ��ʽΪ��H2N-NH2��
��ѹ298Kʱ0.2mol����̬�⻯����O2 ����ȫȼ�գ�������̬A���ʺ�ˮ���ų�����106.8kJ��1mol�������⻯��ų�����Ϊ��106.8kJ��$\frac{1mol}{0.2mol}$=534kJ�������̬�⻯��ȼ���ȵ��Ȼ�ѧ����ʽΪ��N2H4��g��+O2��g��=N2��g��+2H2O��l����H=-534kJ/mol��
�ʴ�Ϊ��H2N-NH2�� N2H4��g��+O2��g��=N2��g��+2H2O��l����H=-534kJ/mol��
��C����ΪF2��B���⻯��ΪH2O��������ˮ��Ӧ�Ļ�ѧ����ʽΪ��2H2O+2F2=4HF+O2��
�ʴ�Ϊ��2H2O+2F2=4HF+O2��
��E2+��D�ļ�����������ͬ�ĵ��Ӳ�ṹ����EΪCaԪ�أ�EB2ΪCaO2���������ӻ�����õ���ʽ��ʾ�����γɹ���Ϊ��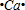 +2
+2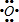 ��
��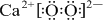 ��
��
�ʴ�Ϊ��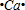 +2
+2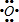 ��
�� ��
��
��NF3��ˮ��ӦΪNF3+2H2O=HNO2+3HF�����NaOH��Һ������ӦΪ��NF3+4NaOH=NaNO2+3NaF+2H2O��
�ʴ�Ϊ��NF3+4NaOH=NaNO2+3NaF+2H2O��
���� ���⿼����λ�á��ṹ�����ʹ�ϵ��Ӧ�ã���Ŀ�Ѷ��еȣ��漰Ԫ���ƶϡ�����ʽ����ѧ����ʽ���Ȼ�ѧ����ʽ����д��֪ʶ��ע������Ԫ�����ڱ��ṹ��Ԫ�����������ݣ�������ؿ���ѧ���ķ������������������Ӧ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 8 | B�� | 10 | C�� | 14 | D�� | 30 |
| A�� | �ڣ��ݣ��ۣ��ܣ��� | B�� | �ݣ��ڣ��ܣ��٣��� | C�� | �ڣ��ݣ��ܣ��ۣ��� | D�� | �ڣ��ۣ��ݣ��٣��� |
| A�� | 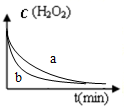 ͼ��ʾ˫��ˮ�ֽ�ʱ��Ӧ��Ũ����ʱ��ı仯�����aΪ��������FeCl3ʱ�ı仯��� | |
| B�� | 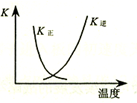 ͼ�����߱�ʾ��Ӧ2SO2��g��+O2��g���T2SO3��g������H��0 �����淴Ӧ��ƽ�ⳣ��K���¶ȵı仯 | |
| C�� | 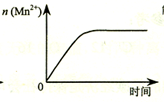 ͼ��ʾ10 mL 0.01 mol•L-1 KMnO4 ������Һ�������0.1 mol•L-1 H2C2O4��Һ���ʱ��n��Mn2+�� ��ʱ��ı仯 | |
| D�� | 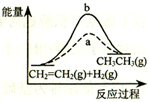 ͼ��a��b���߷ֱ��ʾ��ӦCH2=CH2 ��g��+H2��g����CH3CH3��g������H��0��Ӧ�����е������仯��aΪ���Ӵ���ʱ�Ĺ��� |
| A�� | ��״���£�22.4LCH4��CH2C12�Ļ��������������ΪNA | |
| B�� | �ú�0.2mo1 FeC13�ı�����Һ�Ƶý��壬������������������������ĿΪ0.2 NA | |
| C�� | ��NO2��CO2��ɵĻ�������й���NA�����ӣ�������Ԫ�ص�����һ��Ϊ32g | |
| D�� | 2LpH=1��HA����Һ������п����Ӧ������H2�ķ�����һ��Ϊ0.1 NA |
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 |
��1��д�����ȷ�Ӧ�Ļ�ѧ����ʽ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3��
��2���ӱ��������Ʋ����ȷ�Ӧ���õ����������������Ͻ�����ɣ�Al���۵��Fe�ĵͣ�
��3����������֪ʶд��һ����֤��������Fe����������ô��������������������˵���������ɣ�
��4�����һ����ʵ�鷽����֤���������õ��������к��н���������ֻ�÷�Ӧ���ӷ���ʽ��ʾ����2Al+2OH-+2H2O=2AlO2-+3H2����
��5�������ȷ�Ӧ�У��������õù��࣬�ڷ�Ӧ�ɻ��յ������������Ļ���������15g�û�������150mLϡ�����У��ڱ�״���·ų�1.68L������Ϊ�к��������ᣬ��ʹ��Һ�е�Al3+ǡ����ȫת��ΪAl��OH��3��������Ҫ200mLŨ��Ϊ6mol•L-1��NaOH��Һ��ͬ��ϡ��������ʵ���Ũ����4mol/L��
��ش��������⣺
| B | D | |
| E |

��1��W�ĵ���ʽΪ

��2�����ҷ�Ӧ���ɱ�״����1.12LY������9.025kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽN2��g��+O2��g��=2NO��g����H=+361kJ/mol
��3����ʯī���缫���NaE��Һ�����ӷ���ʽΪ2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$H2��+Cl2��+2OH-
��4����һ������A2��B2�Ļ���������1L�ܱ������У���500�桢2��107Pa�´ﵽƽ�⣮���ƽ������������ʵ���Ϊ0.50mol������A2Ϊ0.3mol��B2Ϊ0.1mol��ƽ��ת����ָijһ���滯ѧ��Ӧ�ﵽƽ��״̬ʱ��ת��ΪĿ�IJ����ij��ԭ����ռ����ԭ����ʼ���İٷ��������������A2��ƽ��ת����Ϊ33.3%��
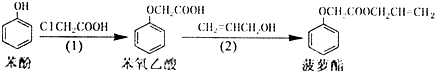
| A�� | ���裨1������2���ķ�Ӧ���Ͳ�ͬ | |
| B�� | ���裨1�������в����ı��ӿ���FeCl3��Һ���� | |
| C�� | ��������Ͳ�����������NaOH��Һ������Ӧ | |
| D�� | ���裨2�������в�����ϩ����������ˮ���� |
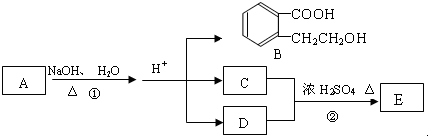
 �������������
�������������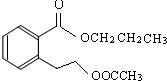 ��
��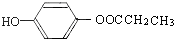 ��
��