��Ŀ����
[���ʽṹ������]
���Բ��ϵ��������Ͻ����Fe(NO3)3��Ni(NO3)2������ͪ뿡��������������������ơ������������һ�������·�Ӧ�Ƶá�
��1�� ��̬Niԭ�ӵļ۵����Ų�ʽ��________��
��2�� ����ͪ�(�ṹ��ʽ����ͼ��ʾ)��̼ԭ�ӵ��ӻ���ʽΪ________��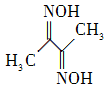
��3�� NH3�ķе����PH3������Ҫԭ����____��
��4�� ��N3�����Ӿ�����ͬ����������ԭ�ӷ��ӵĿռ乹����________��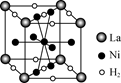
��5�� ��Ni(NO3)2��Һ�еμӰ�ˮ���տ�ʼʱ������ɫNi(OH)2����������ˮ����ʱ���������ܽ⣬����[Ni(NH3)6]2������ɫ��Һ����1 mol[Ni(NH3)6]2�����еĦҼ�Ϊ________mol��
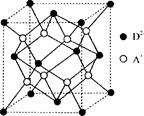
��6�� ��ͼ��һ�������Ͻ����ľ����ṹʾ��ͼ���úϽ����1 mol La�ĺϽ������H2����ĿΪ________��
��1�� 3d84s2��
��2�� sp2��sp3��
��3�� NH3���Ӽ�������
��4�� V�͡�
��5�� 24��
��6�� 3 mol��3NA
���������������2���ṹ��̼ԭ�Ӿ��γɵ�����Ϊsp3�ӻ�����3���������Ӽ����������������ܷе�ߣ���4������Ϊ10����������ԭ�ӷ���Ϊˮ����ԭ��Ϊsp3�ӻ����ռ�ṹΪV�ͣ���5�������е�ԭ����Ni�γ���λ������6����ÿ�������������γ�3�������������6+3��6=24mol��
��6�������ṹ��ԭ����Ŀ��La 8��1/8=1
H 8��1/4+2��1/2=3
���Ժ�1 mol La�ĺϽ������H2����ĿΪ3mol��
���㣺�������ʽṹ�й����⡣

�����Ԫ���ڿ�ѧ�о����������������ŷdz��㷺����;��
��1���������Ľ�������������һ�ܷɻ��������˾Ϳ���̧�������Ԫ�����ڱ��еı�ʾ��ͼ��ʾ����д���¿ո�
��Li��Ԫ�����ڱ��е�λ�ã� ��
��6��941�����壺 __________________________��
��2�����������ü������ܵ�������ʱ���γɵ�����һ���ЧӦ�������Ƴɵġ�
�����蘆�ԭ�ӽṹʾ��ͼ��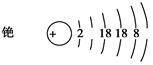
�������й�蘆�˵����ȷ����________��
| A���䵥���ڳ�������ˮ��Ӧ�����ƾ��ң� | B����ԭ�Ӱ뾶�ȼ�ԭ�Ӱ뾶С |
| C�������������ˮ��Һ����ʹ���������ܽ⣬ | D����̼����������ˮ |
(11��)A��B��C��D��E��F���ֶ���������Ԫ�أ����ǵ�ԭ��������������A�ж��ֺ��أ�����һ��û�����ӣ�BԪ�ص�����������Ӧˮ���������⻯���������Σ�D��Aͬ���壬����Eͬ���ڣ�EԪ��ԭ�ӵ��������������������������3/4��A��B��D��E������Ԫ�أ�ÿһ����CԪ�ض����γ�ԭ�Ӹ����Ȳ�ͬ�Ķ��ֻ������ش��������⣺
��1�� F��Ԫ�����ڱ��е�λ����_______________;�õ���ʽ��ʾA��F����Ԫ����ɵĻ������
�γɹ���______________________________________________��
��֪�±��е��������ƻ�1 mol�����еĻ�ѧ�������յ�������kJ����Ԫ��A�ĵ�����Ԫ��F�ĵ�����һ�������·�Ӧ����2mol����ʱ�ͷŵ�����Ϊ____________kJ��
| ��ѧ�� | Cl2 | Br2 | I2 | HCl | HBr | HI | H2 |
| ������kJ�� | 243 | 193 | 151 | 432 | 366 | 298 | 436 |
��������֤E��F����Ԫ�طǽ���ǿ�����ǣ���д��ĸ��
A���Ƚ�������Ԫ�صij������ʵķе�
B���Ƚ�������Ԫ�صĵ������������ϵ�����
C���Ƚ�������Ԫ�ص����������ˮ���������
��3�� A��C��E����֮����γɼס��������������Ǿ�Ϊ��һ����λ����ɵ�˫ԭ�������ӣ��Ҽ���18�����ӣ�����10�����ӣ�������ҷ�Ӧ�����ӷ���ʽΪ