��Ŀ����
20�������ʵ����ʵ���ó�����Ӧ������ȷ���ǣ�������| ʵ����ʵ | ���� | |
| �� | ��ij����ͨ��Ʒ����Һ�У�Ʒ����Һ��ɫ | ������һ����SO2 |
| �� | ��ȼ�յ�þ������CO2���ܼ���ȼ�� | ��ԭ�ԣ�Mg��C |
| �� | NaHCO3��Һ��NaAlO2��Һ��ϲ�����ɫ���� | ���ԣ�HCO3-��Al��OH��3 |
| �� | �����°�����ȼ���������ڷŵ�ʱ����������Ӧ | �ǽ����ԣ�P��N |
| �� | ij��ɫ��Һ�м�������������Һ�����ȣ�������������ʹʪ���ɫʯ����ֽ���� | ����Һ��һ����NH4+ |
| A�� | �٢ڢ� | B�� | �٢ۢ� | C�� | �ڢۢ� | D�� | �ۢܢ� |
���� ��Ʒ����Һ��ɫ���������Ϊ�������������ȣ���϶�������ļ��鷽�������жϣ�
��ȼ�յ�þ������CO2���ܼ���ȼ�գ�����MgO��C��������ԭ��Ӧ�л�ԭ���Ļ�ԭ�Դ��ڻ�ԭ���
��NaAlO2��Һ��ƫ��������ӽ���������������������������ٽ�̼��������ӵĵ��룬���������������������̼������ӣ�
��N��N���ܴ������ȶ���Ӧ���õ��ʺ��������ϵ������������⻯���ȶ��Ի���ۺ�������������ȽϷǽ����ԣ�
�ݲ�����������ʹʪ���ɫʯ����ֽ����������Ϊ������
��� �⣺��Ʒ����Һ��ɫ������Ϊ�������������ȣ������Ȼָ���ɫ������Ϊ�������ʢٴ���
��ȼ�յ�þ������CO2���ܼ���ȼ�գ�����MgO��C���ɻ�ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�Կ�֪����ԭ��ΪMg��C���ʢ���ȷ��
��NaAlO2��Һ�ٽ�̼��������ӵĵ���������������������̼������ӣ������ԣ�HCO3-��Al��OH��3���ʢ���ȷ��
��N��N���ܴ������ȶ������ú��������ϵ������������⻯���ȶ��Ի���ۺ�������������ȽϷǽ����ԣ������°�����ȼ���������ڷŵ�ʱ����������Ӧ����Ϊ���ӽṹ��ԭ����ʵΪ�ǽ�����N��P���ʢܴ���
�ݺͼ���Һ�����Ȳ�����������ʹʪ���ɫʯ����ֽ����������Ϊ��������þ�����һ����NH4+���ʢ���ȷ��
����������֪�ڢۢ���ȷ��
��ѡC��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬�漰���ʵ����ʡ���Ӧ��������۵Ĺ�ϵ�ȣ��������ʵ����ʼ���Ӧԭ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | Oԭ�ӷ���sp�ӻ� | B�� | Oԭ����H��Cl���γɦҼ� | ||
| C�� | �÷���Ϊֱ���ͷ��� | D�� | HClO���ӵĽṹʽ�ǣ�H-Cl-O |
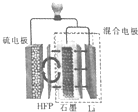 ������ô���¬��ѧ�Ŀ�ѧ�������Li-S����ؼ�����ȡ����һ���ش�ͻ�ƣ�һ�������װ����ͼ��ʾ�����еľ�������ϩ��HFP����Ĥֻ����Li+ͨ������֪�ŵ�ʱ����ܷ�ӦΪLi2S6+10Li=6Li2S������˵����ȷ���ǣ�������
������ô���¬��ѧ�Ŀ�ѧ�������Li-S����ؼ�����ȡ����һ���ش�ͻ�ƣ�һ�������װ����ͼ��ʾ�����еľ�������ϩ��HFP����Ĥֻ����Li+ͨ������֪�ŵ�ʱ����ܷ�ӦΪLi2S6+10Li=6Li2S������˵����ȷ���ǣ�������| A�� | �ŵ�ʱ��Li+���ƶ� | |
| B�� | ���ʱ�������������٣������������� | |
| C�� | �ŵ�ʱ�������ĵ缫��ӦʽΪS62-+10e-=6S2- | |
| D�� | ����LiClˮ��Һ����HFP��Ĥ |
��1��250��ʱ�������Ͻ�Ϊ��������4L������ͨ��6molCO2��6molCH4��������Ӧ��CO2��g��+CH4��g��?2CO��g��+2H2��g����ƽ����ϵ�и�������ʵ��������
| ���� | CH4 | CO2 | CO | H2 |
| ���ʵ���mol | 2 | 2 | 8 | 8 |
����֪��CH4��g��+2O2��g��=CO2��g��+2H2O��g����H=-890.3kJ•mol-1
CO��g��+H2O��g��=CO2��g��+H2��g����H=+2.8kJ•mol-1
2CO��g��+O2��g��=2CO2��g����H=-566.0kJ��mol-1
��ӦCO2��g��+CH4��g��?2CO��g��+2H2��g���ġ�H=+247.3 kJ•mol-1��
��2���Զ������ѱ��渲��Cu2Al2O4Ϊ���������Խ�CO2��CH4ֱ��ת��Ϊ�����ᣮ
���ڲ�ͬ�¶��´����Ĵ�Ч�������������������ͼ1��ʾ��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ�����¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ�
��Ϊ����߸÷�Ӧ��CH4��ת���ʣ����Բ�ȡ�Ĵ�ʩ����С�������ѹǿ������CO2��Ũ�ȣ�
��3����Li2O��Na2O��MgO��������CO2�����Ѱ������CO2���������ʣ����н����������ab��
a�����ڼ�����������Ѱ��
b�����ڢ�A����A��Ԫ���γɵ���������Ѱ��
c���ھ���ǿ�����Ե�������Ѱ��
��Li2O����CO2�������ںϳ�Li4SiO4�������ա��ͷ�CO2��ԭ���ǣ���500�棬CO2��Li4SiO4�Ӵ�������Li2CO3����һ���Σ�ƽ��������700�棬��Ӧ������зų�CO2��Li4SiO4������д��CO2��Li4SiO4��Ӧ�Ļ�ѧ����ʽCO2+Li4SiO4$\frac{\underline{\;500��\;}}{\;}$Li2CO3+Li2SiO3��
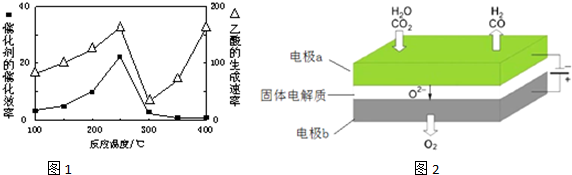
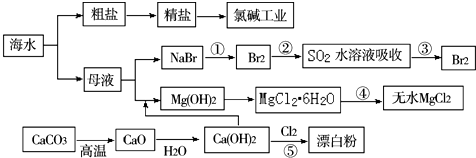
��4����ӦA��CO2+H2O$\frac{\underline{\;���\;}}{����}$CO2+H2+O2���÷�ӦA�ɽ��ͷŵ�CO2ת��Ϊ���й�ҵ���ü�ֵ�IJ�Ʒ�����µ�⼼���ܸ�Чʵ�ַ�ӦA������ԭ��ʾ��ͼ��ͼ2��CO2�ڵ缫a�ŵ�ķ�Ӧʽ��CO2+2e-�TCO+O2-��
| A�� | ����һ��ǿ������������Һ�����������ġ�ͨ·����˵������һ��������ˮ�� | |
| B�� | ��Al��Ӧ�ų�H2����Һ��Mg2-��Ca2-��HCO3-��NO3-�п��ܴ������� | |
| C�� | Ca��ClO��2��Һ��ͨ�����SO2�����ӷ���ʽ��ClO-+SO2+H2O=HClO+HSO3- | |
| D�� | ������������ʵ���Ũ�ȵ�NaX������HX��Ϻ����Һ��c��Na+����c��HX����c��H+����c��OH-�� |
| A�� | Si��P��SԪ�ص�����������ν��� | |
| B�� | Li��Na��K��ԭ�Ӱ뾶�������� | |
| C�� | C��O��N��ԭ�Ӱ뾶���μ�С | |
| D�� | Na��Mg��Alԭ�ӵ��������������μ��� |
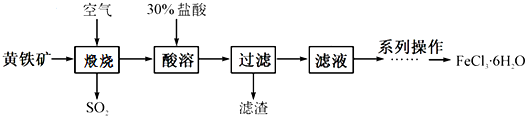
| A�� | Cl2��Ư�ۺ�SO2������Ư����ɫ���ʣ���Ư��ԭ����ͬ | |
| B�� | �ȼҵ����ָ��ҵ�ϵ�ⱥ��ʳ��ˮ�ķ�����ȡNaOH��Cl2��H2�Ĺ��� | |
| C�� | ���ܱ����ڸ����HCl�����м��Ȳ�����ȡ��ˮMgCl2 | |
| D�� | ���٢ڢ۷����ķ�Ӧ��Ϊ������ԭ��Ӧ������Ԫ�ؾ������� |