��Ŀ����
10��ij����С��������Ȳ������KMnO4��Һ��Ӧ�ⶨ��Ȳ����Է�����������ͼ�Dzⶨװ�õ�ʾ��ͼ��B�е�CuSO4���ڳ�ȥ��Ȳ�л��е�H2S��PH3��AsH3�Ȼ�ԭ�����壮
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪCaC2+2H2O��Ca��OH��2+HC��CH����Ϊ�õ�ƽ�ȵ���Ȳ������Һ��a�DZ���ʳ��ˮ��
��2������ƽC2H2������KMnO4��Һ��Ӧ�Ļ�ѧ����ʽ���ں����������������
1C2H2+2KMnO4+3H2SO4=1K2SO4+2MnSO4+2CO2��+4H2O
��3��ʵ��ǰD�к���x mol��KMnO4��ʵ��ʱ��D��ͨ��һ��������Ȳֱ������KMnO4��Һǡ����ȫ��ɫ��ʵ����Ϻ�װ��D��E��������������y g����ͨ���������������Ȳ����Է������������ú���ĸx��y�Ĵ���ʽ��ʾ����$\frac{2y}{x}$����д������̣���
��4������С���ʵ��ԭ�������в�������ȷ�����������У���������Ȳ��Է���������ֵ�������Ӱ����ǣ�C��
A����װ��A�в����Ļ������ֱ��ͨ��D�е�����KMnO4��Һ
B����Eװ�ã�ʢ�м�ʯ�ҵĸ���ܣ�����ʢ��Ũ�����ϴ��ƿ
C��ͨ�������������Ȳ����������KMnO4��Һʱ���в�����Ȳδ���������ݳ�
��5����һ�С���ͬѧ��Ϊ����ʵ��װ���Դ��ڲ��㣬�����ü�Ҫ������˵���Ľ��Ĵ�ʩ��Eװ�ú����ٽ�һ��ʢ�м�ʯ�ҵĸ���ܣ�
���� ��1��ʵ�����õ�ʯ��ˮ��Ӧ�Ʊ���Ȳ��̼������ˮ��Ӧ�����������ƺ���Ȳ����Ӧ�ܾ��ң��ñ���ʳ��ˮ���Լ�����Ӧ��
��2������������ԭ�л��ϼ�����������ȡ������غ������ƽ��
��3�����ݹ�ϵʽ��C2H2��2KMnO4���ɸ�����ص����ʵ��������Ȳ�����ʵ������ٸ���װ��D��E��������������yg������Ȳ����Ϊyg���������Ȳ����Է���������
��4�������Ƿ�Ӱ��װ��D��E��������������
��5��Eװ�������տ����е�ˮ�Ͷ�����̼��
��� �⣺��1��ʵ�����õ�ʯ��ˮ��Ӧ�Ʊ���Ȳ��̼������ˮ��Ӧ�����������ƺ���Ȳ���䷴Ӧ�Ļ�ѧ����Ϊ��CaC2+2H2O��Ca��OH��2+HC��CH����̼������ˮ��Ӧ�ܾ��ң��ñ���ʳ��ˮ���Լ�����Ӧ������Ϊ�˵õ�ƽ�ȵ��������ñ���ʳ��ˮ����ˮ��
�ʴ�Ϊ��CaC2+2H2O��Ca��OH��2+HC��CH��������ʳ��ˮ��
��2��C2H2��̼�Ļ��ϼ���-1���߸�+4�����ϼ۱仯10��KMnO4���̵Ļ��ϼ���+7���͵�+2�����ϼ۱仯5�����ߵ���С������Ϊ10����������غ�ɵã���C2H2+2KMnO4+3H2SO4�TK2SO4+2MnSO4+2CO2��+4H2O��
�ʴ�Ϊ��1��2��3��1��2��2��4��
��3�����ݹ�ϵʽ��n��C2H2��=$\frac{1}{2}$n��KMnO4��=$\frac{1}{2}$xmol����Ȳ����������װ��D��E�������������ص���������m��C2H2��=yg������M=$\frac{m}{n}$=$\frac{yg}{\frac{1}{2}x}$=$\frac{2y}{x}$g/mol������Է�������Ϊ$\frac{2y}{x}$��
�ʴ�Ϊ��$\frac{2y}{x}$��
��4��A����װ��A�в����Ļ������ֱ��ͨ��D�е�KMnO4������Һ���ᵼ����Ȳ������ƫ���������⣻
B����Eװ�ã�ʢ�м�ʯ�ҵĸ���ܣ�����ʢ��Ũ�����ϴ��ƿ��Ũ��������ն�����̼�����»ᵼ����Ȳ������ƫС�����������⣻
C��ͨ�������������Ȳ������KMnO4������Һʱ���в�����Ȳδ���������ݳ����ⶨ�Ǹ��ݸ�����ص�����ȷ����Ȳ��������Ȳ������Ӱ�죬�������⣻
�ʴ�Ϊ��C��
��5��Eװ�������տ����е�ˮ�Ͷ�����̼����ʹ�ⶨ��Eװ������ƫ��������Eװ�ú����ٽ�һ��ʢ�м�ʯ�ҵĸ���ܣ�
�ʴ�Ϊ����Eװ�ú����ٽ�һ��ʢ�м�ʯ�ҵĸ���ܣ�
���� ���⿼���˲ⶨ��Ȳ��Է��������ķ������漰��Ȳ���Ʊ���������ԭ����ʽ��ƽ�����ӣ�Ӱ�����صȣ�����ʵ�����������ʵ����Ʒ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| t/�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK==$\frac{[CO]•[{H}_{2}O]}{[C{O}_{2}]•[{H}_{2}]}$��
��2���÷�ӦΪ���ȷ�Ӧ������ȡ����ȡ�����
��3�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������BC��
A��������ѹǿ����
B�����������C��CO������
C��V��H2����=V��H2O����
D��c��CO2��=c��CO��
��4��ij�¶��£�ƽ��Ũ�ȷ�����ʽ��C��CO2��•C��H2��=c��CO��•c��H2O�������жϴ�ʱ���¶�Ϊ830�森
�����������������ˮ��Һ�мȴ����ܽ�ƽ���ִ��ڵ���ƽ�⣮
��֪Cu��OH��2�TCu2++2OH-��KSP=c��Cu2+��•[c��OH-��]2=2��l0-20������Һ�и�����Ũ�ȷ��εij˻������ܶȻ�ʱ���������������֮�����ܽ⣮
��1��ijCuSO4��Һ��c��Cu2+��=0.02mol•L-1����Ҫ����Cu��OH��2������Ӧ��������pHʹ֮����5��
��2��Ҫʹ0.2mol•L-1CuSO4��Һ��Cu2+������Ϊ��ȫ��ʹCu2+Ũ�Ƚ���ԭ����ǧ��֮һ������Ӧ����Һ�����NaOH��Һ��ʹ��ҺPHΪ6��
| A�� | ${\;}_{y}^{m}$X | B�� | ${\;}_{y}^{y+m}$X | C�� | ${\;}_{y+n}^{m+y+n}$X | D�� | ${\;}_{y+n}^{y+m-n}$X |
��1��A�Ľṹ��ʽΪ
 B�ķ���ʽΪC4H8O3
B�ķ���ʽΪC4H8O3��2��B�����ж���ͬ���칹�壬������һ�ֺ����Ȼ��������������ʼף��÷����ܷ������ַ�Ӧ������������Ԫ��״�����ң�Ҳ���������ʽ������ͬ�����־ۺ�����Ͷ��������ɶ�����Ӧ�õ��ģ�����ֱ�д����Щ���ʵĽṹ��ʽ��
| �� | �� ����Ԫ��״����� | �� �������۷�Ӧ���ɣ� | �� �����Ӿ۷�Ӧ���ɣ� |
 |  |  | 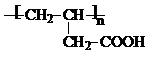 |
| A�� | ������������ԭ��Ӧ | |
| B�� | �����ʵ������������ʲ��������������ͬ״�����Ĵ�С��ϵ�ǣ�Na2O2=CaC2��CaH2=Mg3N2 | |
| C�� | Na2O2��CaH2��ˮ��Ӧʱˮ�������� | |
| D�� | CaH2��ˮ��Ӧʱ����H2��������������������ǻ�ԭ���� |
| A�� | ͬ��������Ԫ�ص�ԭ�Ӱ뾶�Ԣ�A���Ϊ��� | |
| B�� | �����ڱ�������Ԫ�صĵ��ʳ��³�ѹ��ȫ�������� | |
| C�� | ��A����A��Ԫ�ص�ԭ�ӣ���뾶Խ���Խ���õ��� | |
| D�� | �ǽ���Ԫ�ص�����۶���������������� |
| A�� | C4H10 | B�� | C3H8 | C�� | CH4 | D�� | C2H6 |
 ��������BA4�ĵ���ʽΪ
��������BA4�ĵ���ʽΪ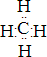 ��
��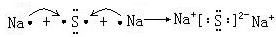 ��
��