��Ŀ����
15��������ij���ʵ�ת����Ϊ���ĵ��ĸ����ʵ���������ռ��ʼʱ�����ʵ�ԭ���ʵ����İٷֱȣ���֪�ϳɰ���ӦΪN2��g��+3H2��g��?2NH3��g������һ���Ϊ10L���ݻ�������ܱ������з���1molN2��3molH2����һ�������·�����Ӧ����4min�ﵽ��ѧƽ��״̬����������а��������ʵ���Ϊ0.6mol��1��������ת����Ϊ30%��
��2��������Ũ�ȱ仯��ʾƽ�ⷴӦ�ٶ���0.0225mol/��L•min����
���� ���ݻ�ѧƽ������ʽ��ʽ���㣬
N2��g��+3H2��g��?2NH3��g��
��ʼ����mol�� 1 3 0
�仯����mol�� 0.3 0.9 0.6
ƽ������mol�� 0.7 2.1 0.6
��1��ת����=$\frac{������}{��ʼ��}$��100%��
��2��V=$\frac{��c}{��t}$���������ķ�Ӧ���ʣ�
��� �⣺��4min�ﵽ��ѧƽ��״̬����������а��������ʵ���Ϊ0.6mol����
N2��g��+3H2��g��?2NH3��g��
��ʼ����mol�� 1 3 0
�仯����mol�� 0.3 0.9 0.6
ƽ������mol�� 0.7 2.1 0.6
��1������ת����=$\frac{������}{��ʼ��}$��100%=$\frac{0.3mol}{1mol}$��100%=30%��
�ʴ�Ϊ��30%��
��2��������Ũ�ȱ仯��ʾƽ�ⷴӦ�ٶ�V=$\frac{��c}{��t}$=$\frac{\frac{0.9mol}{10L}}{4min}$=0.0225mol/��L•min����
�ʴ�Ϊ��0.0225mol/��L•min����
���� ���⿼���˻�ѧƽ������ʽ��ʽ����ķ�����ת���ʡ���Ӧ���ʸ��������Ӧ�úͼ�����������ջ����ǹؼ�����Ŀ�ϼ�

��1��G��Q+NaCl
��2��Q+H20$\stackrel{���}{��}$X+H2
��3��Y+NaOH��G+Q+H2O
��4��Z+NaOH��X+Q+H2O
�����ֻ��������ȵĻ��ϼ��ɵ͵��ߵ�˳���ǣ�������
| A�� | Q G Z Y X | B�� | Z X G Y Q | C�� | G Y Z Q X | D�� | G Y Q Z X |
| A�� | ���������Һ��ͨ�����������̼ Ca2++2ClO-+H2O+CO2�TCaCO3��+2HClO | |
| B�� | ����������Һ�мӹ���������Һ Fe2++2H2O2+4H+�TFe3++4H2O | |
| C�� | �ð�ˮ���������������� 2NH3��H2O+SO2�T2NH4++SO32-+H2O | |
| D�� | ��������Һ�мӹ�����ˮ Fe3++3NH3��H2O�TFe��OH��3��+3NH4+ |
| Ԫ�ش��� | X | Y | Z | M | R | Q | |
| ԭ�Ӱ뾶����10 -10m�� | 1.86 | 0.99 | 1.43 | 1.60 | 0.75 | 0.74 | |
| ��Ҫ���ϼ� | ������� | +1 | +7 | +3 | +2 | +5 | -- |
| ����� | -- | -1 | -- | --- | -3 | -2 | |
| A�� | X��Z��M��������Ӱ뾶��СΪX��M��Z | |
| B�� | X��Z��R������������ˮ����֮�䲻���������Ӧ | |
| C�� | R���⻯���Q���⻯��е�ߡ����ȶ� | |
| D�� | Y���⻯���ˮ��Һ�����ڵ�̲��� |
| A�� | ����������Һ�м���˫��ˮ�������ų���ClO-+H2O2�TO2��+Cl-+H2O | |
| B�� | ̼�������ϡ������Һ��CaCO3+2H+�TCa2++CO2��+H2O | |
| C�� | ��ϡ����ϴ������������Ӧ���Թܣ�Ag+4H++NO3-�TAg++NO��+2H2O | |
| D�� | ��������Һ��ͨ������CO2��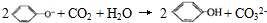 |
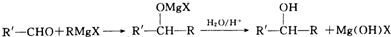
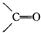 �������
�������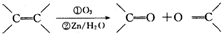
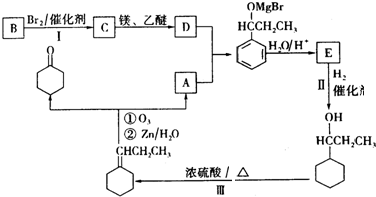
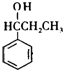 ��
��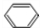 +Br2$\stackrel{����}{��}$
+Br2$\stackrel{����}{��}$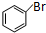 +HBr��
+HBr��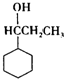 ��
��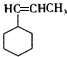 ��F��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽ��n
��F��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽ��n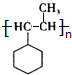 ��
��
 ��
��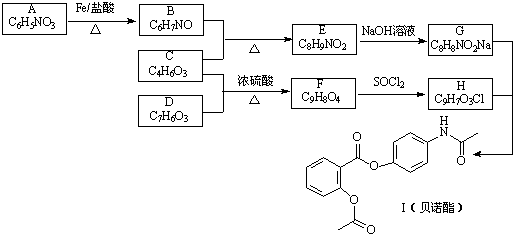

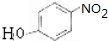 ��G
��G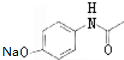 ��
�� ��
�� ��
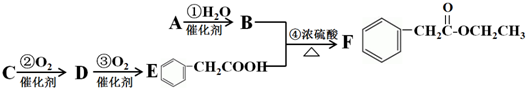
�� �ĺ�����·�������Լ���ѡ��������ͼ��ʾ��д����Ӧ����P��Ҫ��Ӧ������
�ĺ�����·�������Լ���ѡ��������ͼ��ʾ��д����Ӧ����P��Ҫ��Ӧ������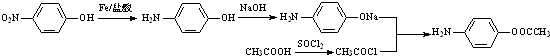 ��
��