��Ŀ����
������������A��B��C��D��E�����ǵ������ӿ�����Na����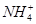 ��Cu2����Ba2����Al3����Ag����Fe3���������ӿ�����Cl����
��Cu2����Ba2����Al3����Ag����Fe3���������ӿ�����Cl����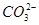 ��
��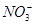 ��
��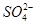 ����֪��
����֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ�ʻ�ɫ��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԡ�
�������������ε���Һ�зֱ����Ba(NO3)2��Һ��ֻ��A��C����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��
�ް�A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����
��ش��������⣺
(1)�������У�һ��û�е��������� ��������������ͬ�������εĻ�ѧʽ�� ��
(2)D�Ļ�ѧʽΪ ��D��Һ�Լ��Ե�ԭ���� (�����ӷ���ʽ��ʾ)��
(3)A��C����Һ��Ӧ�����ӷ���ʽ�� ��
E�Ͱ�ˮ��Ӧ�����ӷ���ʽ�� ��
(4)��Ҫ����B�������������ӣ���ȷ��ʵ�鷽���� ��
(1)Cu2����Fe3��(NH4)2SO4��Al2(SO4)3
(2)Na2CO3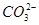 ��H2O
��H2O
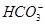 ��OH��
��OH��
(3)Ag����Cl��=AgCl�� Al3����3NH3��H2O=Al(OH)3����3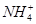
(4)ȡ����B���Թ��У�����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��۲��Ƿ����ɫ
����

 ��У����ϵ�д�
��У����ϵ�д������£����и������ӡ�������ָ����Һ��һ���ܴ����������
| A��Na2S��Һ�� SO42-�� K����Br����Cu2������ |
| B����c(H+)=1.0��10-13mol��L-1����Һ�� Na+��S2����AlO2-��SO32- |
| C����ʹ�����Ժ�ɫ����Һ�� K+��MnO4-��H2C2O4��SO42- |
| D���� NH3��H2O ����Һ��Ba2+��NO3-��Cl-��Ag+ |
��������I��II����ȷ���������ϵ����
| ѡ�� | ����I | ����II |
| A | KNO3���ܽ�ȴ� | ���ؽᾧ����ȥKNO3�л��е�NaCl |
| B | BaSO4�������� | �������BaCl2��Һ����SO42- |
| C | NH3��ʹ��̪��Һ��� | NH3�����������Ȫʵ�� |
| D | Ca(OH)2���Ƴɳ���ʯ��ˮ | ������2.0 mol?L-1��Ca(OH)2��Һ |
��Ƴ���ͭ��ˮ�к���CN-��Cr2O72-����,��Ҫ������������ŷš��ó��ⶨ�������̽��з�ˮ������ �ش��������⣺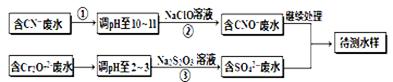
��1������������ˮ��������Ҫʹ�õķ����� ��
��2�����з�Ӧ��������ų����÷�Ӧ�����ӷ���ʽΪ ��
��3��������У�ÿ����0.4mol Cr2O72-ʱת�Ƶ���2.4mol���÷�Ӧ���ӷ���ʽΪ ��
��4��ȡ��������ˮ�����Թ��У�����NaOH��Һ���۲쵽����ɫ�������ɣ��ټ�Na2S��Һ�� ��ɫ����ת���ɺ�ɫ��������ʹ�û�ѧ��������ֽ��Ͳ����������ԭ�� ��
��5��Ŀǰ��������Cr2O72-��ˮ������������巨���÷������ˮ�м���FeSO4��7H2O��Cr2O72-��ԭ��Cr3+������pH��Fe��Crת�����൱�� (��������,�������ֱ�ʾԪ�ؼ�̬)�ij���������1mol Cr2O72-�������a mol FeSO4?7H2O�����н�����ȷ���� ��
(��������,�������ֱ�ʾԪ�ؼ�̬)�ij���������1mol Cr2O72-�������a mol FeSO4?7H2O�����н�����ȷ���� ��
| A��x ="0.5" ,a =8 | B��x ="0.5" ,a = 10 | C��x =" 1.5" ,a =8 | D��x =" 1.5" ,a = 10 |
ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
| ������ | CO32����SiO32����AlO2����Cl�� |
| ������ | Al3����Fe3����Mg2����NH4����Na�� |
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ����(V)�Ĺ�ϵͼ��ʾ��
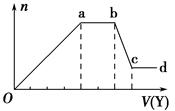
(1)��Y�����ᣬ��Oa��ת��Ϊ����������(ָ��Դ��X��Һ�ģ���ͬ)�� ��
ab�η�����Ӧ�������� ��bc�η�����Ӧ�����ӷ���ʽΪ ��
(2)��Y��NaOH��Һ����X��һ�����е������� ��ab�η�Ӧ�����ӷ���ʽΪ ��
������A��B����ѧ���������ʣ�����������ֻ�ܴ��±���ѡ��
| ������ | K����Na����Fe2����Ba2����NH4+��Ca2�� |
| ������ | OH����NO3����I����HCO3����AlO2����HSO4�� |
(1)��A��B��ˮ��Һ��Ϊ��ɫ����A��ˮ��Һ��ǿ���ԣ�B��ˮ��Һ��ǿ���ԡ���Ϻ����������ϡ����İ�ɫ��������ʹ��ɫʯ����ֽ���������塣
��B�Ļ�ѧʽΪ________________��
��A��B��Һ��ϼ��ȷ�Ӧ�����ӷ���ʽΪ________��
(2)��A��ˮ��Һ��dz��ɫ��B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ����������Ա仯����
��A�Ļ�ѧʽΪ____________________��
�ھ�����������������Һ��Ƶ�ԭ�����������(�����ӷ���ʽ��ʾ)��
i________________________________��ii_______________________________��
������һ������֤��������Һ��Ƶ�ԭ��________��
��������Һ���ԭ����������Ƴ�ԭ��أ���������a����b(a��b��Ϊʯī�缫)����b���ĵ缫��ӦʽΪ____________________��