��Ŀ����
������A��B����ѧ���������ʣ�����������ֻ�ܴ��±���ѡ��
| ������ | K����Na����Fe2����Ba2����NH4+��Ca2�� |
| ������ | OH����NO3����I����HCO3����AlO2����HSO4�� |
(1)��A��B��ˮ��Һ��Ϊ��ɫ����A��ˮ��Һ��ǿ���ԣ�B��ˮ��Һ��ǿ���ԡ���Ϻ����������ϡ����İ�ɫ��������ʹ��ɫʯ����ֽ���������塣
��B�Ļ�ѧʽΪ________________��
��A��B��Һ��ϼ��ȷ�Ӧ�����ӷ���ʽΪ________��
(2)��A��ˮ��Һ��dz��ɫ��B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ����������Ա仯����
��A�Ļ�ѧʽΪ____________________��
�ھ�����������������Һ��Ƶ�ԭ�����������(�����ӷ���ʽ��ʾ)��
i________________________________��ii_______________________________��
������һ������֤��������Һ��Ƶ�ԭ��________��
��������Һ���ԭ����������Ƴ�ԭ��أ���������a����b(a��b��Ϊʯī�缫)����b���ĵ缫��ӦʽΪ____________________��
(1)��Ba(OH)2
��H����SO42����NH4+��Ba2����2OH�� BaSO4����NH3����2H2O
BaSO4����NH3����2H2O
(2)��FeI2
��i.6I����2NO3����8H��=3I2��2NO����4H2O
��.2I����Fe2����NO3����4H��=I2��Fe3����NO����2H2O
(��3Fe2����NO3����4H��=NO����2H2O��3Fe3��)
��ȡ���������Һ���Թ��У��μӼ���KSCN��Һ�������������
��NO3����4H����3e��=NO����2H2O
����

 ����ѧ����ϵ�д�
����ѧ����ϵ�д�ijУͬѧΪ̽��Br2��I2��Fe3����������ǿ��������������ʵ�顣
ʵ��٣�ȡ����KI��Һ���Թ��У��ȼ�����ˮ�����ټ���CCl4�����ã��۲쵽�²�Һ����Ϻ�ɫ��
ʵ��ڣ�ȡ����FeSO4��Һ���Թ��У��ȼ�����ˮ�����ټ����μ�����KSCN��Һ�����۲쵽��Һ�ʺ�ɫ��
(1)д�����ӷ���ʽ��
ʵ��٣�__________________________________________________________��
ʵ��ڣ�__________________________________________________________��
(2)����������ʵ�飬�����ʵ������Կ��Եó�����ȷ������________��
| A��Br2>I2 | B��Fe3��>Br2 |
| C��Br2>Fe3�� | D��I��>Br�� |
��FeCl3��Һ���ڵ�ˮ����KI��Һ����ϡH2SO4���ݵ�����Һ
__________________________________________________________________
__________________________________________________________________
���Ŵ�����Ⱦ���������أ��������ڡ�ʮ�������ڼ䣬����������(SO2)�ŷ�������8%����������(NOx)�ŷ�������10%��Ŀǰ������������Ⱦ�ж��ַ�����
��1����CH4����ԭ��������������������������Ⱦ����֪��
��CH4(g)+4NO2(g) ="4NO(g)" + CO2(g) +2H2O(g) �SH=" -574" kJ��mol��1
��CH4(g) +4NO(g) =2N2(g) + CO2(g) + 2H2O(g) �SH=" -1160" kJ��mol��1
��H2O(g) = H2O(l) ��H=" -44.0" kJ��mol��1
д��CH4(g)��NO2(g)��Ӧ����N2 (g)��CO2 (g)��H2O(1)���Ȼ�ѧ����ʽ ��
��2������Fe2+��Fe3+�Ĵ����ã������¿ɽ�SO2ת��ΪSO42-���Ӷ�ʵ�ֶ�SO2����������֪��SO2�ķ���ͨ�뺬Fe2+��Fe3+����Һʱ������һ����Ӧ�����ӷ���ʽΪ4Fe2+ + O2+ 4H+ = 4Fe3+ + 2H2O������һ��Ӧ�����ӷ���ʽΪ ��
Ũ��/mol��L��1
| NO | N2 | CO2 | ||
| 0 | 1.00 | 0 | 0 | ||
| 10 | 0.58 | 0.21 | 0.21 | ||
| 20 | 0.40 | 0.30 | 0.30 | ||
| 30 | 0.40 | 0.30 | 0.30 | ||
| 40 | 0.32 | 0.34 | 0.17 | ||
| 50 | 0.32 | 0.34 | 0.17 |
��3���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g)
 N2 (g)+CO2 (g) ��ij�о�С�����ܱյ���������У���������������䣬��������������Բ��ƣ�����NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2 (g)+CO2 (g) ��ij�о�С�����ܱյ���������У���������������䣬��������������Բ��ƣ�����NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£���10min~20min��v(CO2) ��ʾ��ƽ����Ӧ����Ϊ ��
�ڸ��ݱ������ݣ�����T1��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ ���� ��������λС������
��һ���¶��£�����NO����ʼŨ��������NO��ƽ��ת���� ������������䡱��С���� ��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽƽ����� ������ ���������ĸ����
A��������ѹǿ���ֲ���
B��2v��(NO) = v��(N2)
C��������CO2�������������
D�����������ܶȱ��ֲ���
��30minĩ�ı�ijһ��������һ��ʱ�䷴Ӧ���´ﵽƽ�⣬��ı������������ ������ͼ�л���30min��40min�ı仯���ߡ�

ij�������Ƹ﹤ҵ������Cr�������������ù������£������ȡҺ�н���������Ҫ��
Cr3���������Fe3����Al3����Ca2����Mg2������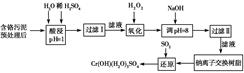
| �������� | Fe��OH��3 | Mg��OH��2 | Al��OH��3 | Cr��OH��3 |
| pH | 3.7 | 11.1 | 8 | 9����9��Һ�� |
��1�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩΪ________________������дһ������
��2����pH��8��Ϊ�˳�ȥ________����Fe3����Al3����Ca2����Mg2������ͬ����
��3�������ӽ�����֬��ԭ��ΪMn����nNaR�D��MRn��nNa����������������������________��
��4������ƽ��Ӧ����ʽ��

 ��
������1 mol Cr��OH����H2O��5SO4����SO2�����ʵ���Ϊ________��
��֪Ca(OH)2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ(�����ķ�Ӧ��Ϊ���ȷ�Ӧ)���������к���Cl����ClO����ClO3�����ֺ���Ԫ�ص����ӣ�����ClO����ClO3���������ӵ����ʵ���(n)�뷴Ӧʱ��(t)��������ͼ��ʾ��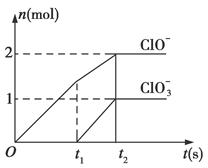
(1)t1ǰ������������______________________(�ѧʽ)��
(2)t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵ����ӷ���ʽ��_____________________________��
(3)��ʯ�����к���Ca(OH)2�����ʵ�����________mol��
(4)NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ��������________(����ĸ)��
| A��NaCl��Cl2 | B��NaCl��NaClO | C��NaClO3��NaClO4 | D��NaCl��NaClO3 |
��.���ǵؿ��к�����ߵĽ���Ԫ��,�䵥�ʼ���Ͻ������������е�Ӧ��ʮ�ֹ㷺��
(1)����������������Al2O3Ϊԭ��,�����ʯ(Na3AlF6)������״̬�½��е��,��ѧ����ʽΪ ����
��缫����ʯī��������,����ʱ�������ĵĵ缫����������(���������������)��
(2)������Ʒ���п���ʴ����,���ӳ���ʹ���������Դ�����������Ϊ����,��H2SO4��Һ�е��,���ı����γ�����Ĥ,������ӦʽΪ�� ��
(3)�����������Խ,Al-Ag2O��ؿ�����ˮ�¶�����Դ,��ѧ��ӦΪ:2Al+3Ag2O+2NaOH+3H2O 2Na[Al(OH)4]+6Ag, ���ĵ缫��ӦʽΪ
2Na[Al(OH)4]+6Ag, ���ĵ缫��ӦʽΪ
��������������������,����������Һ��pH��������(���������䡱��С��)��
��.���ǵ����Ϻ����ḻ��һ��Ԫ��,�����仯�����ڹ�ũҵ������������������Ҫ���á�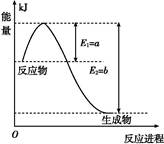
(1)��ͼ����һ���¶Ⱥ�ѹǿ��N2��H2��Ӧ����1 mol NH3�����������仯ʾ��ͼ,��д���ϳɰ����Ȼ�ѧ��Ӧ����ʽ: (��H����ֵ�ú���ĸa��b�Ĵ���ʽ��ʾ)��
(2)��ҵ�ϳɰ��ķ�ӦΪN2(g)+3H2(g) 2NH3(g)����һ���¶���,��һ������N2��H2ͨ�����Ϊ1 L���ܱ�������,��Ӧ�ﵽƽ���,�ı���������,��ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ���������������
2NH3(g)����һ���¶���,��һ������N2��H2ͨ�����Ϊ1 L���ܱ�������,��Ӧ�ﵽƽ���,�ı���������,��ʹƽ��������Ӧ�����ƶ���ƽ�ⳣ���������������
| A������ѹǿ | B������Ӧ���Ũ�� |
| C��ʹ�ô��� | D�������¶� |
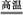 6SO2+Fe3O4,��3 mol FeS2�μӷ�Ӧ,ת����������mol���ӡ�
6SO2+Fe3O4,��3 mol FeS2�μӷ�Ӧ,ת����������mol���ӡ�(2)�Ȼ�����Һ��Ϊ��ѧ�Լ��еġ������֡�,д��SO2ͨ���Ȼ�����Һ�з�Ӧ�����ӷ���ʽ:�� ��
 ��Cu2����Ba2����Al3����Ag����Fe3���������ӿ�����Cl����
��Cu2����Ba2����Al3����Ag����Fe3���������ӿ�����Cl���� ��
�� ��
�� ����֪��
����֪��