��Ŀ����
11����ѧ����1978���Ƶ�һ������A��A�ɿ�������B��������ԭ�ӱ���C��һ�ۻ�ȡ�����ã�A��Br2��CCl4��Һ����ɫ��A����ԭ�ӱ�1����ԭ��ȡ��ֻ��һ�����ʣ�һ������C��ȫȼ�����õ�H2O��CO2�����ʵ���֮��Ϊ1.25��1��C��ͬ���칹�岻����3�֣���C�Ķ������Ϊ3�֣�һ������B��ȫȼ�����ɵ�CO2��H2O�����ʵ���֮��Ϊ2��B����Է�����������26��С��78���Իش��������⣺��1��B�ķ���ʽΪC4H4��
��2��д��C��3�ֶ������Ľṹ��ʽ��
 ��
�� ��
��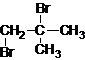 ��
����3��A�ķ���ʽΪC20H36��A�Ľṹ��ʽΪ
 ��
��
���� B��ȫȼ�յIJ�����n��CO2����n��H2O��=2��1����B����ɿ��Ա�ʾΪ��CH��n������26��M��A����78����26��13n��78�����2��n��6��B��̼ԭ�Ӹ�������Ϊ3��4��5����������ԭ�Ӹ���Ϊż������B�ķ���ʽֻ��ΪC4H4��C��ȫȼ�գ����õ�H2O��CO2�����ʵ���֮��Ϊ 1.25��1����C��n��C����n��H��=1��2.5����������ԭ�Ӹ���Ϊż������������⡢̼ԭ��������Ϊ��2n+2����n����֪C�ķ���ʽΪC4H10���DZ���������A�ɿ�������B��������ԭ�ӱ���C��һ�ۻ�ȡ�����ã�A��Br2��CCl4��Һ����ɫ��A�е���ԭ�ӱ�һ����ԭ��ȡ��ֻ�ܵõ�һ���ȴ����˵��������Hԭ��Ϊ��ЧHԭ�ӣ�C��ͬ���칹�岻����3�֣���C�Ķ������Ϊ3�֣�����CΪ��CH��CH3��3��C��һ������Ϊ-C��CH3��3������AΪ �������ʽΪC20H36���ݴ˷������
�������ʽΪC20H36���ݴ˷������
��� �⣺B��ȫȼ�յIJ�����n��CO2����n��H2O��=2��1����B����ɿ��Ա�ʾΪ��CH��n������26��M��A����78����26��13n��78�����2��n��6��B��̼ԭ�Ӹ�������Ϊ3��4��5����������ԭ�Ӹ���Ϊż������B�ķ���ʽֻ��ΪC4H4��C��ȫȼ�գ����õ�H2O��CO2�����ʵ���֮��Ϊ 1.25��1����C��n��C����n��H��=1��2.5����������ԭ�Ӹ���Ϊż������������⡢̼ԭ��������Ϊ��2n+2����n����֪C�ķ���ʽΪC4H10���DZ���������A�ɿ�������B��������ԭ�ӱ���C��һ�ۻ�ȡ�����ã�A��Br2��CCl4��Һ����ɫ��A�е���ԭ�ӱ�һ����ԭ��ȡ��ֻ�ܵõ�һ���ȴ����˵��������Hԭ��Ϊ��ЧHԭ�ӣ�C��ͬ���칹�岻����3�֣���C�Ķ������Ϊ3�֣�����CΪ��CH��CH3��3��C��һ������Ϊ-C��CH3��3������AΪ �������ʽΪC20H36��
�������ʽΪC20H36��
��1��ͨ�����Ϸ���֪��B�ķ���ʽΪC4H4���ʴ�Ϊ��C4H4��
��2��CΪ��CH��CH3��3��C�Ķ������ṹ��ʽΪ

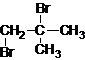


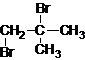
��3��ͨ�����Ϸ���֪��A�ķ���ʽΪC20H36���ṹ��ʽΪ���ʴ�Ϊ��C20H36��
���� ���⿼���л����ƶϣ����ؿ���ѧ�������ƶϼ���ȡ��Ϣ������Ϣ��������ȷ�ж�B�ṹ��ʽ�ǽⱾ��ؼ�����Ŀ�Ѷ��еȣ�

 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д� ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�| A�� | X��Y���ܶ��ǹ��� | B�� | X��Y������һ�������� | ||
| C�� | X��Y���ܶ������� | D�� | X��Yһ���������� |
| A�� | WX2����������ԭ������㶼Ϊ8 ���ӽṹ | |
| B�� | WX2��ZX2�Ļ�ѧ��������ͬ | |
| C�� | WX2���Լ��Լ���ϳɵķ��� | |
| D�� | ԭ�Ӱ뾶��С˳��ΪX��W��Y��Z |
| A�� | �ϳɰ���Ӧ��N2��g��+3H2��g��?2NH3��g������H��0��Ϊʹ���IJ�����ߣ�������Ӧ��ȡ���¸�ѹ�Ĵ�ʩ | |
| B�� | ��2HI��g��?H2��g��+I2��g��ƽ����ϵ����ѹǿʹ��ɫ���� | |
| C�� | SO2��������SO3�ķ�Ӧ��������Ҫʹ�ô��� | |
| D�� | ��֪��Ӧ2NO2��g��?N2O4��g��������ɫ����NO2��ѹ����ɫ�ȱ�����dz |
| A�� | H2O2�ĵ���ʽ��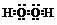 | B�� | ��ԭ�ӵĽṹʾ��ͼ��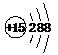 | ||
| C�� | �Ҵ��Ľṹʽ��C2H6O | D�� | ����Ľṹ��ʽ��C2H4O2 |
 ��
�� ���þ����к�����������У����Ӽ��ͷǼ��Լ���
���þ����к�����������У����Ӽ��ͷǼ��Լ���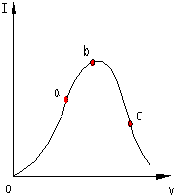 ��һ���¶��£��������ˮϡ�����У���Һ�ĵ�������I�����ˮ�����V�仯��������ͼ��ʾ����ش�
��һ���¶��£��������ˮϡ�����У���Һ�ĵ�������I�����ˮ�����V�仯��������ͼ��ʾ����ش�