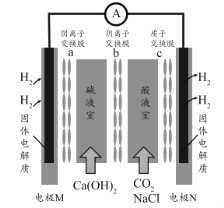
��Ŀ����
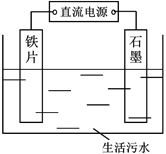
����Ŀ��������ˮ�еĵ�������Ҫ����κ���������ʽ���ڣ����õ�ⷨ����Һ��ȥ�������װ����ͼ��������������ʯī���������ɽ��г�������ת��Դ��������������������ʯī���������ɽ��г��ס�
I��������
��1���ڼ�����Һ�У�NH3��ֱ���ڵ缫�ŵ磬ת��ΪN2����Ӧ�ĵ缫��ӦʽΪ��_______��
��2����Cl-����ʱ������ԭ����ͼ1��ʾ����Ҫ������Ч�ȣ�HClO��ClO-����NH4+ ��NH3����ΪN2���ڲ�ͬpH�����½��е��ʱ������ȥ���ʺ�ˮ����Ч��Ũ����ͼ2��
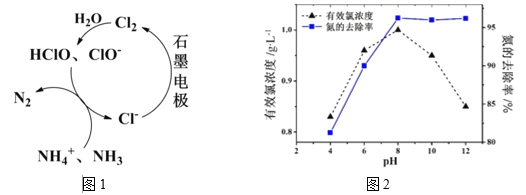
�ٵ�pH<8ʱ����Ҫ����HClO����NH4+ �ķ�Ӧ�������ӷ���ʽΪ��____________��
�ڽ��ƽ���ƶ�ԭ�����ͣ���pH<8ʱ������ȥ������pH�Ľ��Ͷ��½���ԭ���ǣ�_____��
�۵�pH>8ʱ��ClO-�����绯������Ч��Ũ���½���������ȥ����ȴ��δ�����½������ܵ�ԭ���ǣ����һ�㼴�ɣ���______��
II��������
��3������ԭ��������Fe2+ ��PO43- ת��ΪFe3(PO4)2������
���û�ѧ�����ʾ����Fe2+����Ҫ���̣�_______________��
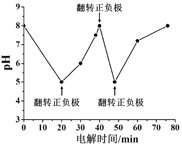
����ͼΪij��Cl- ��ˮ�ڵ��������ѳ���������ҺpH�ı仯���Ʋ���20-40 minʱ�ѳ���Ԫ����________��
��4���ⶨ��ˮ�����ķ������£�ȡ100mL��ˮ������������pH����AgNO3��Һʹ��ȫ��ת��ΪAg3PO4���������������˲�ϴ�Ӻ��������ܽ⣬��ʹ��NH4SCN��Һ�ζ�������Ag+��������ӦAg++SCN-=AgSCN����������c mol/LNH4SCN��ҺV mL�������ˮ���ĺ���Ϊ___mg/L������Ԫ�ؼƣ���
���𰸡�2NH3�C6e-+6OH-=N2+6H2O 3HClO +2NH4+=3Cl-+N2+3H2O+5H+ ����ҺpH���ͣ�c(H+)����Cl2 + H2O![]() H+ + Cl- +HClOƽ�������ƶ�����Һ��c(HClO)��С��ʹNH4+���������½� pH����������NH4+ת��ΪNH3��NH3��ֱ���ڵ缫�Ϸŵ����������pH����������NH4+ת��ΪNH3����������NH3�ݳ��� Fe�C2e-=Fe2+ ��
H+ + Cl- +HClOƽ�������ƶ�����Һ��c(HClO)��С��ʹNH4+���������½� pH����������NH4+ת��ΪNH3��NH3��ֱ���ڵ缫�Ϸŵ����������pH����������NH4+ת��ΪNH3����������NH3�ݳ��� Fe�C2e-=Fe2+ �� ![]()
��������
��1��������Һ�У�NH3ת��ΪN2�����ϼ۽��͵õ��ӣ�
��2����pH<8ʱ��HClO��NH4+ ����ΪN2������ҺpH���ͣ�c(H+)����Cl2 + H2O![]() H+ + Cl- +HClOƽ�������ƶ�����pH>8ʱ��������NH4+ת��ΪNH3��NH3��ֱ���ڵ缫�Ϸŵ����������������NH3�ݳ���
H+ + Cl- +HClOƽ�������ƶ�����pH>8ʱ��������NH4+ת��ΪNH3��NH3��ֱ���ڵ缫�Ϸŵ����������������NH3�ݳ���
��3������ʱ��Fe������ʧ���ӣ���������pH���С�������ף�pH������
��4���ɹ�ϵʽP~ Ag3PO4~ 3AgSCN~3NH4SCN���
��1���ڼ�����Һ�У�NH3ת��ΪN2�����ϼ۽��͵õ��ӣ���Ӧ�ĵ缫��ӦʽΪ2NH3�C6e-+6OH-=N2+6H2O��
��2���ٵ�pH<8ʱ��HClO��NH4+ ����ΪN2�����ӷ���ʽΪ3HClO +2NH4+=3Cl-+N2��+3H2O+5H+��
�ڵ�pH<8ʱ������ҺpH���ͣ�c(H+)����Cl2 + H2O![]() H+ + Cl- +HClOƽ�������ƶ�����Һ��c(HClO)��С��ʹNH4+���������½�����ȥ������pH�Ľ��Ͷ��½���
H+ + Cl- +HClOƽ�������ƶ�����Һ��c(HClO)��С��ʹNH4+���������½�����ȥ������pH�Ľ��Ͷ��½���
�۵�pH>8ʱ��ClO-�����绯������Ч��Ũ���½���NH4+���������½���������ȥ����ȴ��δ�����½������ܵ�ԭ����pH����������NH4+ת��ΪNH3��NH3��ֱ���ڵ缫�Ϸŵ����������pH����������NH4+ת��ΪNH3����������NH3�ݳ�����
��3���ٳ���ʱ��Fe������ʧ���ӣ�����Fe2+����Ҫ����ΪFe�C2e-=Fe2+��
����ͼ��ҺpH�ı仯���ڼ�����Һ�У�������ʱ2NH3![]() N2+3H2������NH3��pH���С�������ף������������������������ӣ�pH������20-40 minʱpH�������ѳ���Ԫ�����ף�
N2+3H2������NH3��pH���С�������ף������������������������ӣ�pH������20-40 minʱpH�������ѳ���Ԫ�����ף�
��4���ɹ�ϵʽP��Ag3PO4��3AgSCN��3NH4SCN��֪��n��P��=![]() n��NH4SCN��=
n��NH4SCN��=![]() cV
cV![]() mol�������ˮ���ĺ���Ϊ��
mol�������ˮ���ĺ���Ϊ��![]() cV
cV![]() mol
mol![]() 0.1L=
0.1L=![]() cV mg/L��
cV mg/L��