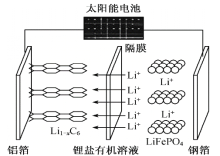
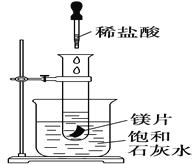
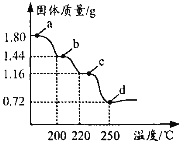
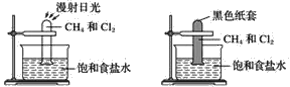
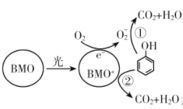
��Ŀ����
����Ŀ����֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
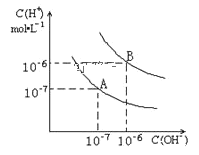
��1��25��ʱ��ˮ�ĵ���ƽ������ӦΪ_________��˵������_________
��2��95��ʱ����pH=9��NaOH��Һ��pH=4��������Һ��ϣ������û����Һ��pH=7����NaOH��Һ��������Һ�������Ϊ_________
��3��95��ʱ����10���pH1=a��ijǿ����Һ��1���pH2=b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ��_________
���𰸡�A ˮ�ĵ�����һ�����ȹ��̣��¶Ƚ���ʱ������̶ȼ�С����ˮ�����ӻ���С 1:9 pH1+ pH2=13
��������
��1������A������Kw=c(H+)![]() c(OH-)=107��107=1014,����B������Kw= c(H+)
c(OH-)=107��107=1014,����B������Kw= c(H+)![]() c(OH-)106��106=1012��ˮ�ĵ�����һ�����ȹ��̣��¶Ƚ���ʱ������̶ȼ�С����ˮ�����ӻ���С���ʴ�Ϊ��A��ˮ�ĵ�����һ�����ȹ��̣��¶Ƚ���ʱ������̶ȼ�С����ˮ�����ӻ���С��
c(OH-)106��106=1012��ˮ�ĵ�����һ�����ȹ��̣��¶Ƚ���ʱ������̶ȼ�С����ˮ�����ӻ���С���ʴ�Ϊ��A��ˮ�ĵ�����һ�����ȹ��̣��¶Ƚ���ʱ������̶ȼ�С����ˮ�����ӻ���С��
��2��95��ʱˮ�����ӻ�Ϊ1012,���û����Һ��pH=7,��Һ�ʼ��ԣ�����c(OH-)=![]() =10-5�����
=10-5�����![]() =1:9���ʴ�Ϊ��1:9��
=1:9���ʴ�Ϊ��1:9��
��3��95��ʱˮ�����ӻ�Ϊ1012, pH2=b��ijǿ����Һ��c(OH-)=![]() =10b-12 mol/L����10���pH1=a��ijǿ����Һ��1���pH2=b��ijǿ����Һ��Ϻ���Һ�����ԣ���n(H+)=n(OH-)����10-a
=10b-12 mol/L����10���pH1=a��ijǿ����Һ��1���pH2=b��ijǿ����Һ��Ϻ���Һ�����ԣ���n(H+)=n(OH-)����10-a ![]() 10=10b-12
10=10b-12![]() 1�����a+b=13������ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ��pH1+ pH2=13��
1�����a+b=13������ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ��pH1+ pH2=13��

����Ŀ�����ܱ������з�����![]() ����֪:c(CO2)���¶�(T)��ʱ��(t)�ı仯������ͼ��ʾ����:
����֪:c(CO2)���¶�(T)��ʱ��(t)�ı仯������ͼ��ʾ����:
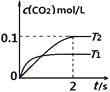
��1��T1____________T2 �� ��������____________
��2����T2�¶��£�0��2s�ڵ�ƽ����Ӧ����v(N2)=____________
��3���������������жϸ÷�Ӧ��ƽ��״̬����____________
A. ƽ����ϵ������ѹǿ���ٸı� B. ƽ����ϵ�������ܶȲ��ٸı�
C. c(CO2):c(N2)=2:1 D. V��(CO):V��(N2)=2:1
��4�����������ݣ����ȣ�����ʹ�������¶�Ѹ������ԭ����2�����ﵽ��ƽ����������¶�________(������������С��������������)ԭ����2����
��5�����¶�T3�£������������Ϊ1.0L�ĺ����ܱ������з����÷�Ӧ.
������� | ��ʼ���ʵ��� | ƽ��ʱ���ʵ��� |
�� | 2.0molNO��2.0molCO | 1.0molCO2 |
�� | 4.0molNO��4.0molCO |
��T3ʱ�÷�Ӧ��ƽ�ⳣ��K=_________������ʼʱ���������г���1.0mol NO��1.5mol CO��2.0molCO2�� 0.5molN2�� ��Ӧ��_________(����������������)��Ӧ������С�
��ƽ��ʱ��������CO��ת���ʣ���_________��,�������з�Ӧ��ƽ�����Ҫ��һ�����CO��ת���ʣ��ɲ�ȡ�Ĵ�ʩΪ(��д������)_________________ __________________��