��Ŀ����
��15�֣��Ի�����Ϊԭ����������Ĺ�������ͼ���£�
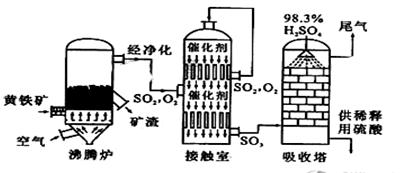
��1��д��ȼ�ջ�����Ļ�ѧ����ʽ ��
����6mol SO2����ʱ��ת�Ƶ��� mol��
��2������Ӵ��ҵ������к���״̬��1120m3SO2���壬��ƽ���ų�����Ϊ4.728��106kJ����ʱSO2ת����Ϊ96%���÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3�����ݹ�������ͼ�ж�����˵����ȷ���ǣ�ѡ�������ĸ�� ��
a��Ϊʹ��������ȼ�գ��轫�����
b���������������SO2��ת����
c��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
d������¯�ų��Ŀ����ɹ�����
e����������ˮ�����ռ�
��4��ij���᳧�ڽ��л�����ɷֲⶨʱ��ȡ0.1000 g��Ʒ������գ����ɵ�SO2����������Fe2(SO4)3��Һ��ȫ��Ӧ������0.02000 mol��L-1��K2Cr2O7����Һ�ζ����յ㣬����K2Cr2O7��Һ25.00mL��
��֪��SO2��2Fe3+��2H2O=SO42����2Fe2+��4H+
Cr2O72����6Fe2+��14H��=2Cr3+��6Fe3+��7H2O
����Ʒ��FeS2�����������Ƕ��٣����������ʲ��μӷ�Ӧ��
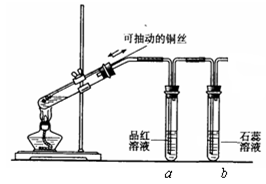
��5�������������в�����β�����˺���N2��O2�⣬������SO2������SO3�������������ڲⶨ����β����SO2�������� ��(����ĸ)

��1��д��ȼ�ջ�����Ļ�ѧ����ʽ ��
����6mol SO2����ʱ��ת�Ƶ��� mol��
��2������Ӵ��ҵ������к���״̬��1120m3SO2���壬��ƽ���ų�����Ϊ4.728��106kJ����ʱSO2ת����Ϊ96%���÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3�����ݹ�������ͼ�ж�����˵����ȷ���ǣ�ѡ�������ĸ�� ��
a��Ϊʹ��������ȼ�գ��轫�����
b���������������SO2��ת����
c��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
d������¯�ų��Ŀ����ɹ�����
e����������ˮ�����ռ�
��4��ij���᳧�ڽ��л�����ɷֲⶨʱ��ȡ0.1000 g��Ʒ������գ����ɵ�SO2����������Fe2(SO4)3��Һ��ȫ��Ӧ������0.02000 mol��L-1��K2Cr2O7����Һ�ζ����յ㣬����K2Cr2O7��Һ25.00mL��
��֪��SO2��2Fe3+��2H2O=SO42����2Fe2+��4H+
Cr2O72����6Fe2+��14H��=2Cr3+��6Fe3+��7H2O
����Ʒ��FeS2�����������Ƕ��٣����������ʲ��μӷ�Ӧ��
��5�������������в�����β�����˺���N2��O2�⣬������SO2������SO3�������������ڲⶨ����β����SO2�������� ��(����ĸ)
| A��NaOH��Һ����̪��Һ | B��KMnO4��Һ��ϡH2SO4 |
| C����ˮ��������Һ | D����ˮ����̪ |
��1��4FeS2 + 11O2 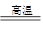 2Fe2O3+8SO2 ��2�֣� 33��2�֣�
2Fe2O3+8SO2 ��2�֣� 33��2�֣�
��2��2SO2(g) �� O2(g) �� 2SO3(g) ���SH����197 kJ/mol ��2�֣�
��3��a b d ����1�֣�
��4�� 3FeS2 �� 2Cr2O72��
3��120 2
m(FeS2) 0.0200��25.00��10-3
m(FeS2)= =0.09000g
=0.09000g
w(FeS2)=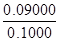 ��100%=90.0% ��4�֣� ��5��BC (2��)
��100%=90.0% ��4�֣� ��5��BC (2��)
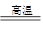 2Fe2O3+8SO2 ��2�֣� 33��2�֣�
2Fe2O3+8SO2 ��2�֣� 33��2�֣���2��2SO2(g) �� O2(g) �� 2SO3(g) ���SH����197 kJ/mol ��2�֣�
��3��a b d ����1�֣�
��4�� 3FeS2 �� 2Cr2O72��
3��120 2
m(FeS2) 0.0200��25.00��10-3
m(FeS2)=
 =0.09000g
=0.09000gw(FeS2)=
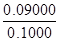 ��100%=90.0% ��4�֣� ��5��BC (2��)
��100%=90.0% ��4�֣� ��5��BC (2��)��1��������ȼ������SO2��������������ʽΪ4FeS2 + 11O2 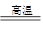 2Fe2O3+8SO2 ����Ӧ����������������ת�Ƶ�����44�����Ե���6mol SO2����ʱ��ת�Ƶ���44mol��8��6��33mol��
2Fe2O3+8SO2 ����Ӧ����������������ת�Ƶ�����44�����Ե���6mol SO2����ʱ��ת�Ƶ���44mol��8��6��33mol��
��2����״̬��1120m3SO2������50000mol�����Է�Ӧ�����ĵ�SO2��50000mol��0.96��48000mol������2molSO2��Ӧ�ų���������4.728��106kJ��24000��197 kJ/mol�������Ȼ�ѧ����ʽΪ2SO2(g) �� O2(g) �� 2SO3(g) ���SH����197 kJ/mol��
��3�������������Ӧ��ĽӴ�������ӿ췴Ӧ���ʣ�a��ȷ������������Ũ�ȿ������SO2��ת���ʣ�b��ȷ���������ܸı�ƽ��״̬��ֻ�ܸı䷴Ӧ���ʣ�c����ȷ��d��ȷ���������ڣ���Ũ�������գ���ѡabd��
��4��������ݹ�ϵʽ���е��йؼ��㡣�����йط���ʽ��֪
3FeS2 �� 2Cr2O72��
3��120 2
m(FeS2) 0.0200��25.00��10-3
m(FeS2)= =0.09000g
=0.09000g
w(FeS2)=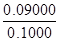 ��100%=90.0%
��100%=90.0%
��5��SO2���л�ԭ�ԣ�����BC��ȷ��AD����ȷ����Ϊ�������������Ҳ�ܱ����ա���ѡBC��
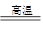 2Fe2O3+8SO2 ����Ӧ����������������ת�Ƶ�����44�����Ե���6mol SO2����ʱ��ת�Ƶ���44mol��8��6��33mol��
2Fe2O3+8SO2 ����Ӧ����������������ת�Ƶ�����44�����Ե���6mol SO2����ʱ��ת�Ƶ���44mol��8��6��33mol����2����״̬��1120m3SO2������50000mol�����Է�Ӧ�����ĵ�SO2��50000mol��0.96��48000mol������2molSO2��Ӧ�ų���������4.728��106kJ��24000��197 kJ/mol�������Ȼ�ѧ����ʽΪ2SO2(g) �� O2(g) �� 2SO3(g) ���SH����197 kJ/mol��
��3�������������Ӧ��ĽӴ�������ӿ췴Ӧ���ʣ�a��ȷ������������Ũ�ȿ������SO2��ת���ʣ�b��ȷ���������ܸı�ƽ��״̬��ֻ�ܸı䷴Ӧ���ʣ�c����ȷ��d��ȷ���������ڣ���Ũ�������գ���ѡabd��
��4��������ݹ�ϵʽ���е��йؼ��㡣�����йط���ʽ��֪
3FeS2 �� 2Cr2O72��
3��120 2
m(FeS2) 0.0200��25.00��10-3
m(FeS2)=
 =0.09000g
=0.09000gw(FeS2)=
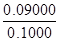 ��100%=90.0%
��100%=90.0%��5��SO2���л�ԭ�ԣ�����BC��ȷ��AD����ȷ����Ϊ�������������Ҳ�ܱ����ա���ѡBC��

��ϰ��ϵ�д�
�����Ŀ
 H2SO4
H2SO4 SO2
SO2 H2SO4
H2SO4