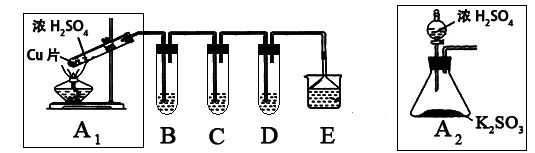
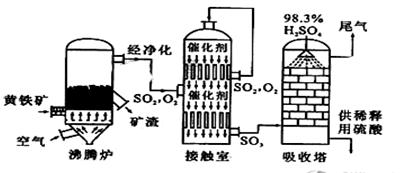
��Ŀ����
����������ת��;����ijЩ��Ӧ�����Ͳ�����ʡ�ԣ������й�˵������ȷ����
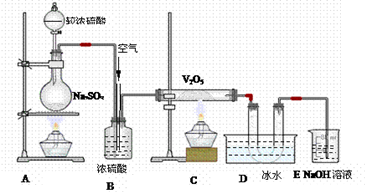
;���١�S H2SO4
H2SO4
;���ڡ�S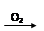 SO2
SO2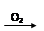 SO3
SO3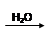 H2SO4
H2SO4
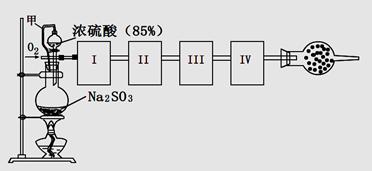
;���١�S
 H2SO4
H2SO4;���ڡ�S
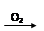 SO2
SO2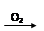 SO3
SO3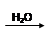 H2SO4
H2SO4| A��;���ٷ�Ӧ��������ŨHNO3��ǿ�����Ժ����� |
| B��;���ڵĵڶ�����Ӧ��ʵ�������п���ͨ������O2Ũ�������ͳɱ� |
| C����;���ٺ͢ڷֱ���ȡ1 mol H2SO4�������ϸ�����1 mol S����ת��6 mol e�� |
| D��;������;������ȸ������֡���ɫ��ѧ������������Ϊ;���ڱ�;������Ⱦ���С��ԭ�������ʸ� |
A
���������;���ٷ�Ӧ��ֻ������ŨHNO3��ǿ�����ԣ�û�����ԣ�����A��������ѡ�����ȷ����ѡA��
����������dz�������

��ϰ��ϵ�д�
 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ