��Ŀ����
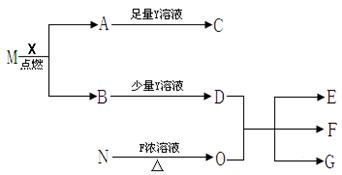
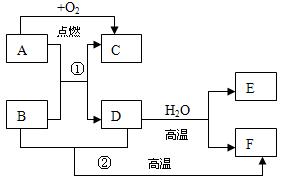
��12�֣��ɶ�����Ԫ����ɵ�A��B��C��D��E��F������������ֻ��C��D�Ƿ��ӣ��������������ӣ���ÿ�����ж�����10�����ӡ���֪A��E���ɷǽ���Ԫ����ɵ������ӣ��������������й�ϵ��
��A��B���������ڼ��������¿�����C��D���ַ��ӣ�
��ͨ��״����C�ľۼ�״̬Ϊ��̬���ҿ�ʹʪ��ĺ�ɫʯ����ֽ������
��1 mol B������1 mol E�������ÿ�����2 mol D���ӣ�
����F���ӵ���Һ�м���C����Һ�������ɰ�ɫ����W��C��Һ��������Ҳ����ʧ�����ټ��뺬����B���ӻ����E���ӵ���Һ������W�����ܽ⡣
(1) ��A�Ļ�ѧʽ��_____________����E��������___________________��������F��Ԫ����Ԫ�����ڱ��е�λ����____________________________��
(2) д��F�����C��Һ��Ӧ�����ӷ���ʽ��___________________ ______
(3) д����C��ˮ��Һ��[Cu(NH3)4]SO4��H2O�����������Ҫ���ӷ�Ӧ����ʽ��
��A��B���������ڼ��������¿�����C��D���ַ��ӣ�
��ͨ��״����C�ľۼ�״̬Ϊ��̬���ҿ�ʹʪ��ĺ�ɫʯ����ֽ������
��1 mol B������1 mol E�������ÿ�����2 mol D���ӣ�
����F���ӵ���Һ�м���C����Һ�������ɰ�ɫ����W��C��Һ��������Ҳ����ʧ�����ټ��뺬����B���ӻ����E���ӵ���Һ������W�����ܽ⡣
(1) ��A�Ļ�ѧʽ��_____________����E��������___________________��������F��Ԫ����Ԫ�����ڱ��е�λ����____________________________��
(2) д��F�����C��Һ��Ӧ�����ӷ���ʽ��___________________ ______
(3) д����C��ˮ��Һ��[Cu(NH3)4]SO4��H2O�����������Ҫ���ӷ�Ӧ����ʽ��
(1) NH4+ ˮ�������� �������ڡ���A��
��2��Al3����3NH3��H2O��Al(OH)3����3NH4��
��3��Cu2++2NH3��H2O��Cu(OH)2����2NH4�� Cu(OH)2+4NH3=[Cu(NH3)4]2++2OH-
��2��Al3����3NH3��H2O��Al(OH)3����3NH4��
��3��Cu2++2NH3��H2O��Cu(OH)2����2NH4�� Cu(OH)2+4NH3=[Cu(NH3)4]2++2OH-
���������10�����ӵ�������NH4++OH-=NH3+H2O��A�������ӣ�����A��NH4+��B��OH-��CΪ��̬���ҿ�ʹʪ��ĺ�ɫʯ����ֽ������C��NH3��D��H2O��1 mol B������1 mol E�������ÿ�����2 mol D���ӣ�E��H3O+����F���ӵ���Һ�м���C����Һ�������ɰ�ɫ����W��C��Һ��������Ҳ����ʧ�����ټ��뺬����B���ӻ����E���ӵ���Һ������W�����ܽ⣬��FΪAl3+��WΪAl(OH)3��
�Ʊ�[Cu(NH3)4]SO4��H2O���壬Ӧ��CuSO4��Һ��ͨ��NH3���Ȳ�����ɫ������������ܽ⣬�������ɫ��Һ���䷢�������ӷ�ӦΪCu2++2NH3��H2O��Cu(OH)2����2NH4�� Cu(OH)2+4NH3=[Cu(NH3)4]2++2OH-��
������10���������ƶ����Ǹ����и߿���һ���ȵ㣬Ҫ��������10�������Ӷ�����Щ����Ҫ�˽����з�����������Ӧ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 �� ��
�� �� ��
��  _________
_________ ����ط������������
����ط������������  __�������ƣ���
__�������ƣ���