��Ŀ����
��15�֣���֪AΪһ����̬��������Է�������С��30��1molA��ȫȼ�����ɵ�CO2��H2O���ʵ���֮��Ϊ1:1���м����C��������һ��Ҳ�ܸ����Ƶ�Cu(OH)2������Ӧ����ש��ɫ������CҲ�ܷ���������Ӧ��E����ζ��FΪһ�־ۺ������Ӧ����δд������
��ش��������⣺
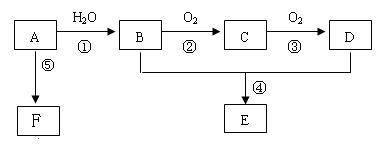
��1������A�ͼ�����Բ��õļ�����������������ͽ��ۣ��У�__________________
��
��2��A����B�ķ�Ӧ����Ϊ_______________��C�Ľṹ��ʽΪ��______________________
B��D�й����ŵ����Ʒֱ���_____________��______________��
��3������B���Ա�ֱ������ΪD����Ҫ������Լ� �� ��
�� ��
��4����Ӧ�ܵĻ�ѧ����ʽ Ϊ��
��  _________
_________
��Ӧ���ͣ� ��
��5����ʵ�����л�õ�E���������Ǻ���B���ʺ�D���ʵĴֲ�Ʒ��Ҫ���ý�Ϊ������E���ʿ��Լ�����Լ��� ����ط������������
����ط������������  __�������ƣ���
__�������ƣ���
��6����Ӧ�ݵĻ�ѧ����ʽΪ��____________________________________________________
��Ӧ���ͣ� ��

��ش��������⣺
��1������A�ͼ�����Բ��õļ�����������������ͽ��ۣ��У�__________________
��
��2��A����B�ķ�Ӧ����Ϊ_______________��C�Ľṹ��ʽΪ��______________________
B��D�й����ŵ����Ʒֱ���_____________��______________��
��3������B���Ա�ֱ������ΪD����Ҫ������Լ�
 �� ��
�� ����4����Ӧ�ܵĻ�ѧ����ʽ
 ��
��  _________
_________��Ӧ���ͣ� ��
��5����ʵ�����л�õ�E���������Ǻ���B���ʺ�D���ʵĴֲ�Ʒ��Ҫ���ý�Ϊ������E���ʿ��Լ�����Լ���
 ����ط������������
����ط������������  __�������ƣ���
__�������ƣ�����6����Ӧ�ݵĻ�ѧ����ʽΪ��____________________________________________________
��Ӧ���ͣ� ��
��15�֣���1��������ֱ�ͨ����������Һ������ˮ������ʹ��Һ��ɫ������ϩ����һΪ���飨���߽�����ֱ��ȼ���۲������ɫ�����������������̵�����ϩ������ɫ���Ǽ��飩������������Ҳ���֣���2�֣�
��2���ӳɷ�Ӧ��CH3CHO���ǻ����Ȼ�����1�֣���3������KMnO4��K2Cr2O7��Һ��1�֣�
��4����CH3COOH+ CH3CH2 OH
OH CH3COOCH2CH3 + H2O ��2�֣� ������Ӧ��ȡ����Ӧ��1�֣� ��5������Na2CO3��Һ ��
CH3COOCH2CH3 + H2O ��2�֣� ������Ӧ��ȡ����Ӧ��1�֣� ��5������Na2CO3��Һ �� Һ ��ÿ�ո�1�֣�
Һ ��ÿ�ո�1�֣�
��6�� (2��) �Ӿ۷�Ӧ��1�֣�
(2��) �Ӿ۷�Ӧ��1�֣�
��2���ӳɷ�Ӧ��CH3CHO���ǻ����Ȼ�����1�֣���3������KMnO4��K2Cr2O7��Һ��1�֣�
��4����CH3COOH+ CH3CH2
 OH
OH CH3COOCH2CH3 + H2O ��2�֣� ������Ӧ��ȡ����Ӧ��1�֣� ��5������Na2CO3��Һ ��
CH3COOCH2CH3 + H2O ��2�֣� ������Ӧ��ȡ����Ӧ��1�֣� ��5������Na2CO3��Һ �� Һ ��ÿ�ո�1�֣�
Һ ��ÿ�ո�1�֣���6��
 (2��) �Ӿ۷�Ӧ��1�֣�
(2��) �Ӿ۷�Ӧ��1�֣���

��ϰ��ϵ�д�
 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д�
�����Ŀ
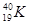 ��
��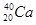
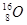 ��
��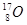 ��
��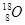
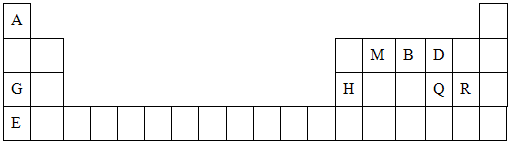

 �밴Ҫ����գ���1��д��A�ĵ���ʽ ��FԪ�������ڱ�λ�� ��2����Ӧ�ݵĻ�ѧ����ʽΪ�� ��
�밴Ҫ����գ���1��д��A�ĵ���ʽ ��FԪ�������ڱ�λ�� ��2����Ӧ�ݵĻ�ѧ����ʽΪ�� �� ���û�������CA3��A2D���۵��ɸߵ��͵�˳��Ϊ ���û�ѧʽ��ʾ����
���û�������CA3��A2D���۵��ɸߵ��͵�˳��Ϊ ���û�ѧʽ��ʾ����