��Ŀ����
12��Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ��±�Ϊ8��Ԫ�������ڱ��е�λ�ã�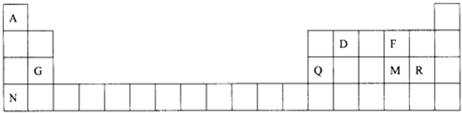
��1����ͼ��ʾ��ģ�ͱ�ʾ�ķ����У�����A��D�γɵ���acd��
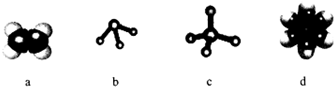
д��c���ӵĿռ乹��Ϊ�������壬d���ӵĽṹ��ʽ
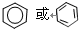 ��
����2������a����ϩ���������������⣺
�����a�����е�һ����ԭ�ӱ���ȡ����õ�����������ͬһƽ���ԭ�������7����
��a��ʹ���ˮ��Һ��ɫ���÷�Ӧ���������������1��2-�������飨��������
��a�����������ӳɷ�Ӧ�����ɷ���e��e�ڷ�����ɺͽṹ�����Ƶ��л�����һ���ࣨ�ֳơ�ͬϵ��������Ǿ�����ͨʽCnH2n+2����n=4_ʱ�������л��↑ʼ����ͬ���칹�壬д�������������е�ͬ���칹��ṹ��ʽCH3CH2CH2CH3����CH3��2CHCH3��
��3��Na��R�ĵ���������ȼ�ղ���ĵ���ʽΪ
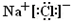 ��Na��F������ȼ�յIJ�����Na2O2��
��Na��F������ȼ�յIJ�����Na2O2����4������Ԫ�ص�����������ˮ�����У�������ǿ����KOH������Q�ĵ��ʷ�Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O=2AlO-2+3H2����
���� ����Ԫ���������ڱ��е�λ�ÿ�֪AΪHԪ�أ�DΪCԪ�أ�FΪOԪ�أ�GΪMgԪ�أ�QΪAlԪ�أ�MΪSԪ�أ�RΪClԪ�أ�NΪKԪ�أ�
��1��������ϩ�ĽṹΪƽ���ͷ��ӣ�����Ϊ�������壻����Ϊ�����Σ���Ϊƽ���ͷ��ӣ����Ľṹ��ʽΪ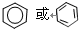 ��
��
��2�����ڳ������л��������м�������������ṹ����ϩ��ƽ���ͽṹ�������л�����ڴ˻����Ͻ����жϣ�
����ϩ����ˮ�����ӳɷ�Ӧ����1��2-�������飻
�۸��������м��顢���顢����û��ͬ���칹�壬�Ӷ��鿪ʼ����ͬ���칹�壬����̼���칹����дͬ���칹�壻
��3����Na��Cl2��O2��Ӧ�IJ����жϣ�
��4����Ԫ�ؽ����Եĵݱ���ɽ��з����������������ط�Ӧ����ƫ����غ�������
��� �⣺����Ԫ���������ڱ��е�λ�ÿ�֪AΪHԪ�أ�DΪCԪ�أ�FΪOԪ�أ�GΪMgԪ�أ�QΪAlԪ�أ�MΪSԪ�أ�RΪClԪ�أ�NΪKԪ�أ�
�⣺��1����ϩ�ĽṹΪƽ���ͷ��ӣ�����Ϊ�������壻����Ϊ�����Σ���Ϊƽ���ͷ��ӣ�����a��c��d����̼��������Ԫ���γɣ�b��N��H����Ԫ���γɣ�
c���ӵĿռ乹��Ϊ�������壻���Ľṹ��ʽΪ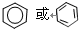 ��
��
�ʴ�Ϊ��acd���������壻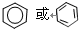 ��
��
��2���ټ�������������ṹ�������3��ԭ�ӹ��棬��ϩ��ƽ���ͽṹ�������6��ԭ�ӹ��棬����a�����е�һ����ԭ�ӱ���ȡ����õ������ʱ�ϩ����ͬһƽ���ԭ�������7����
�ʴ�Ϊ��7��
����ϩ����ˮ�����ӳɷ�Ӧ����1��2-�������飬
�ʴ�Ϊ��1��2-�������飻
�۸��������м��顢���顢����û��ͬ���칹�壬�Ӷ��鿪ʼ����ͬ���칹�壬����n=4�������ͬ���칹���У�CH3CH2CH2CH3����CH3��2CHCH3��
�ʴ�Ϊ��4��CH3CH2CH2CH3����CH3��2CHCH3��
��3��Na��Cl2��ȼ������NaCl���Ȼ���Ϊ���ӻ��������ʽΪ��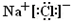 ��Na��O2��ȼ������Na2O2��
��Na��O2��ȼ������Na2O2��
�ʴ�Ϊ��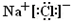 ��Na2O2��
��Na2O2��
��4����������ǿ��Ԫ��Ϊ�������ڢ�A��Ԫ��K����Ӧ����������ˮ����KOH�ļ�����ǿ����Al��Ӧ�����ӷ���ʽΪ
2Al+2OH-+2H2O=2AlO-2+3H2����
�ʴ�Ϊ��KOH��2Al+2OH-+2H2O=2AlO-2+3H2����
���� ���⿼����Ԫ�����ڱ���Ԫ��������Ӧ�ã��漰����ʽ����д���ӳɷ�Ӧ��ԭ�ӹ��������֪ʶ�㣬֪���������ƵĽṹ������ݼ������ϩȷ��ԭ�ӹ��棬Ϊ�״��㣮

 Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�| A�� | ̼������������θ�����˵�θ�����֢ | |
| B�� | �����ƾ���ǿ�Ļ�ԭ�ԣ��������ƺ�TiCl4��Һ��Ӧ��ȡ����Ti | |
| C�� | ���ࡢ��֬����������һ�������¾�����ˮ�� | |
| D�� | ������ˮ��Һ�ʼ��ԣ����������ƹ��� |
| A�� | ${\;}_{17}^{37}$Cl2��Ħ��������74 | |
| B�� | ${\;}_{17}^{37}$Cl��${\;}_{17}^{35}$Cl��Ϊͬλ�أ�${\;}_{17}^{35}$Cl2��Cl2��Ϊͬ���칹�� | |
| C�� | ͨ������£���������������������Ҳ���л�ԭ�� | |
| D�� | ��ʹʪ��ĵ���KI��ֽ����ɫ������һ����Cl2 |
| A�� | ��ϩ������8���Ҽ���1 ���м� | |
| B�� | ��ϩ������3��̼ԭ�Ӷ���sp3�ӻ� | |
| C�� | ��ϩ���Ӳ����ڷǼ��Լ� | |
| D�� | ��ϩ������3��̼ԭ�ӿ�����ͬһֱ���� |
| A�� | 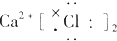 | B�� | 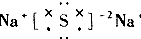 | C�� | 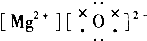 | D�� | 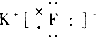 |
| A�� | �ƹ�̼�����ʹ洢��������ʵ�ֶ�����̼���ŷ� | |
| B�� | �Թ�ҵ��ˮ��������ˮ����������������Ⱦ����ŷ� | |
| C�� | �Ӵ�Ǧ���ء�����п�̸ɵ�ص������������������� | |
| D�� | �Ӵ������Դ�Ŀ������ã������Դ�������� |
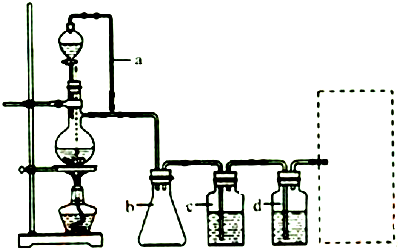
 ��Ȳ���л��ϳɹ�ҵ��һ��ԭ�ϣ���ҵ������CaC2��ˮ��Ӧ������Ȳ��
��Ȳ���л��ϳɹ�ҵ��һ��ԭ�ϣ���ҵ������CaC2��ˮ��Ӧ������Ȳ�� ��1mol O22+�к��еĦм���ĿΪ2NA����
��1mol O22+�к��еĦм���ĿΪ2NA����