��Ŀ����
����Ŀ��NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
��1��NH4Al(SO4)2������ˮ������������_____________________________(�ñ�Ҫ�Ļ�ѧ������������˵��)��
��2����ͬ�����£�0.1 mol��L��1NH4Al(SO4)2��c(![]() )________0.1 mol��L��1NH4HSO4��c(
)________0.1 mol��L��1NH4HSO4��c(![]() )��(����������������������������)
)��(����������������������������)
��3������ʽ��ʾ0.1 mol��L��1NH4Al(SO4)2��Һ�и�����Ũ�ȹ�ϵ____________��
��4����ͼ��0.1 mol��L��1�������Һ��pH���¶ȱ仯��ͼ��
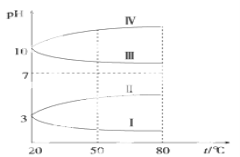
���з���0.1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯��������________(��д��ĸ)������pH���¶ȱ仯��ԭ����_____________________________��
��5������ʱ����100 mL 0.1 mol��L��1HCl��Һ�еμ�0.1 mol��L��1��ˮ���õ���ҺpH�백ˮ����Ĺ�ϵ������ͼ��ʾ��

���Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶�������____________��
����b�㣬��Һ�и�����Ũ���ɴ�С������˳����__________________;
��д��a������Һ��������ʽ�ľ�ȷ��������ܽ��Ƽ��㣩��
c��Cl-��- c��NH4+��=____________��c��H+��- c��NH3��H2O��=____________��
���𰸡�A13+ˮ�����ɵ�A1(OH)3������������ԣ���A13++3H2O= A1(OH)3(����)+3H+��A1(OH)3����������������ʹ������Ӷ�����ˮ С�� c(NH4+)+3c(Al3+)+c(H+)=2c(SO42-)+c(OH-) 1 NHAl(SO4)2ˮ�⣬��Һ�����ԣ������¶���ˮ��̶�����pH��С a c(Cl-)= c(NH4+)��c(OH-)=c(H+) 10-6-10-8�� 10-8
��������
(1)Al3+ˮ�����ɵ�Al(OH)3���壬���������ԣ�
(2)NH4Al(SO4)2��Al3+ˮ�����������NH4+ˮ�⣬HSO4-�����H+ͬ������NH4+ˮ�⣻
(3)��Һ�д��ڵ���غ������
(4)NH4Al(SO4)2ˮ�⣬��Һ�����ԣ������¶���ˮ��̶�����
(5)��a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a������Ͱ�ˮǡ����ȫ��Ӧ����Һ��ֻ��NH4Cl��b��c��d������Һ������NH3H2O���ݴ˷����жϣ���b����Һ�к���NH4Cl��NH3H2O�������ԣ�c(OH-)=c(H+)����ϵ���غ������𣻢�a��Ϊ�Ȼ����Һ�����ݵ���غ�������غ���㡣
(1)Al3+ˮ�����ɵ�Al(OH)3���壬�������������������������ԣ��ܹ�����ˮ���������ʴﵽ��ˮ��Ŀ�ģ�ˮ������ӷ���ʽΪ��Al3++3H2O=Al(OH)3����+3H+���ʴ�Ϊ��Al3+ˮ�����ɵ�Al(OH)3���壬���������ԣ���Al3++3H2O=Al(OH)3����+3H+��Al(OH)3������������ʹ������Ӷ�����ˮ��
(2)NH4Al(SO4)2��NH4HSO4�е�NH4+������ˮ�⣬����NH4Al(SO4)2��Al3+ˮ������Ի�����NH4+ˮ�⣬HSO4-�����H+ͬ������NH4+ˮ�⣬��ΪHSO4-�������ɵ�H+Ũ�ȱ�Al3+ˮ�����ɵ�H+Ũ�ȴ�����NH4HSO4��NH4+ˮ��̶ȱ�NH4Al(SO4)2�е�С���ʴ�Ϊ��С�ڣ�
(3)���ݵ���غ㣬��c(NH4+)+3c(Al3+)+c(H+)=2c(SO42-)+c(OH-)���ʴ�Ϊ��c(NH4+)+3c(Al3+)+c(H+)=2c(SO42-)+c(OH-)��
(4)NH4Al(SO4)2ˮ�⣬��Һ�����ԣ�pH��7�������¶ȣ�ˮ��̶�����pH��С�����ϵ�����Ϊ�ʴ�Ϊ����NH4Al(SO4)2ˮ�⣬��Һ�����ԣ������¶ȣ���ˮ��̶�����pH��С��
(5)��a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a������Ͱ�ˮǡ����ȫ��Ӧ����Һ��ֻ��NH4Cl��b��c��d������Һ������NH3H2O��NH4Clˮ��ٽ�ˮ�ĵ��룬��NH3H2O����ˮ�ĵ��룬���ˮ�ĵ���̶�������a�㣬�ʴ�Ϊ��a��
��b����Һ�к���NH4Cl��NH3H2O�������ԣ�c(OH-)=c(H+)�����ݵ���غ㣬c(NH4+)= c(Cl-)����b��ʱc(Cl-)= c(NH4+)��c(OH-)=c(H+)���ʴ�Ϊ��c(Cl-)= c(NH4+)��c(OH-)=c(H+)��
��a��Ϊ�Ȼ����Һ�����ݵ���غ㣬c(Cl-)- c(NH4+)= c(H+)-c(OH-)=10-6-![]() =10-6-10-8�����������غ�c(H+)=c(NH3��H2O)+ c(OH-)�����c(H+)-c(NH3��H2O)= c(OH-)=10-8���ʴ�Ϊ��10-6-10-8��10-8��
=10-6-10-8�����������غ�c(H+)=c(NH3��H2O)+ c(OH-)�����c(H+)-c(NH3��H2O)= c(OH-)=10-8���ʴ�Ϊ��10-6-10-8��10-8��