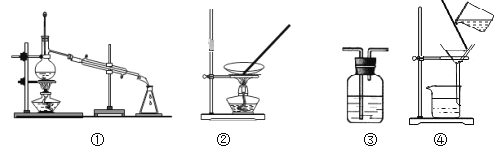
��Ŀ����
����Ŀ����A��B��C��D���ֳ����Ľ������ʣ�A�ڿ�����ȼ�����ɵ���ɫ���壻BΪ��ɫ���壬��ʴʱ��Ϊ��ɫ��C�ڿ����м����ۻ��������䣻D��������ȼ�գ��������䡣
����������Ϣ�ش��������⣺
(1)д����Ӧ��ѧʽ��A______��B______��C______��D______��
(2)A����������������Ӧʱ����__________��д��ѧʽ����ͬ����D�ڿ�������ʴ����__________��
(3)д�����л�ѧ����ʽ��
��A�ڿ�����ȼ��______________________��
��B�������ڼ��������·�Ӧ______________________��
��C��������ȼ��________________________��
���𰸡�Na Cu Al Fe Na2O Fe2O3 2Na��O2![]() Na2O2 2Cu��O2
Na2O2 2Cu��O2![]() 2CuO 4Al��3O2
2CuO 4Al��3O2![]() 2Al2O3
2Al2O3
��������
A��B��C��D���ֳ����Ľ������ʣ�A�ڿ�����ȼ�����ɵ���ɫ���壬����A��Na��BΪ��ɫ���壬��ʴʱ��Ϊ��ɫ������B��Cu��C�ڿ����м����ۻ��������䣬��C��Al��D��������ȼ�գ��������䣬����D��Fe���ݴ˽��
�������Ϸ�����֪A��Na��B��Cu��C��Al��D��Fe����
��1��A��B��C��D�������ʵĻ�ѧʽ�ֱ���Na��Cu��Al��Fe��
��2��A�ǽ����ƣ�����������������Ӧʱ���������ƣ���ѧʽΪNa2O��D�������ڿ�������ʴ��������������ѧʽΪFe2O3��
��3�������ڿ�����ȼ�����ɹ������ƣ�����ʽΪ2Na��O2![]() Na2O2��
Na2O2��
��ͭ�������ڼ��������·�Ӧ��������ͭ������ʽΪ2Cu��O2![]() 2CuO��
2CuO��
������������ȼ������������������ʽΪ4Al��3O2![]() 2Al2O3��
2Al2O3��

����Ŀ��2SO2(g)+ O2(g) ![]() 2SO3(g)�ǹ�ҵ���������Ҫ��Ӧ֮һ��һ���¶��£��ڼס��ҡ��������ݻ���Ϊ2L�ĺ����ܱ�������Ͷ��SO2(g)��O2(g)������ʼ���ʵ�����SO2��ƽ��ת�������±���ʾ�������ж���ȷ����
2SO3(g)�ǹ�ҵ���������Ҫ��Ӧ֮һ��һ���¶��£��ڼס��ҡ��������ݻ���Ϊ2L�ĺ����ܱ�������Ͷ��SO2(g)��O2(g)������ʼ���ʵ�����SO2��ƽ��ת�������±���ʾ�������ж���ȷ����
�� | �� | �� | ||
��ʼ���ʵ��� | n(SO2)/mol | 0.4 | 0.8 | 0.8 |
n(O2)/mol | 0.24 | 0.24 | 0.48 | |
SO2��ƽ��ת����/% | 80 | ��1 | ��2 |
A. ���з�Ӧ��ƽ�ⳣ��С����
B. ���¶��£�ƽ�ⳣ��ֵΪ400
C. ƽ��ʱ������c(SO3)�Ǽ��е�2��
D. ƽ��ʱ������O2��ת���ʴ�������O2��ת����