��Ŀ����
����Ŀ��BaCl2��xH2O����;�㷺�Ļ���������Ʒ���ҹ�Ŀǰ��Ҫ�����������(������Mg2����Fe3����)��Ӧ����BaCl2��xH2O������������ͼ��ʾ����ش�

��֪������ʱKsp[Mg(OH)2]=1.8��10��11��Ksp[Fe(OH)3]=4.0��10��38
��1����ӦI�����ɵ�H2S��������ˮ���գ�һ����������������Һ��ͨ�˿������ֿɵõ�������ʹ����Һ������������Ӧ�Ļ�ѧ����ʽΪ_________��
��2�������Ȼ�����Һ�к�����(H2S��HS����)Ӱ���Ʒ�������ɹ���Ԥ�Ⱥ�Ŀ���������Ԥ�ȿ�����Ŀ����_________������A����Ҫ�ɷ���_________��
��3���ȿ�������ʱ���в���HS��ת��ΪS2O32����ʹ��Ʒ�Բ��ܴﵽ����Ҫ�������ữ�����ữ����ʱ�����ӷ���ʽΪ_________��
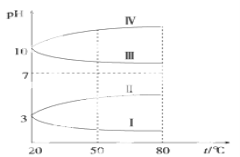
��4������ʱ��ΪʹMg2����Fe3����ȫ����(����Һ������Ũ��С��1��l0��5mol![]() ʱ��Ϊ��������ȫ����)��Ӧ����Һ��pH����_________(ֻ����ʽ)���ϡ�
ʱ��Ϊ��������ȫ����)��Ӧ����Һ��pH����_________(ֻ����ʽ)���ϡ�
��5��ʵ���Ҳⶨ��Ʒ��x�IJ������£�
��ȷ��ȡ12.23 g BaCl2��xH2O��Ʒ������l00 mLϡ��������ܽ⣻
���߽��裬����μ���0.lmol![]() H2SO4��Һ����BaSO4��ȫ���������ˣ�������ϴ�ӳ���2��3�Σ�
H2SO4��Һ����BaSO4��ȫ���������ˣ�������ϴ�ӳ���2��3�Σ�
����������ָ�������������Ϊ11.65 g������BaSO4�����Ƿ�ϴ�Ӹɾ��ķ�����_______��������x����ֵΪ_________��
���𰸡�2(NH4)2S ��O2��2H2O = 4NH3��H2O��2S�� ���½�ʹ������ܽ�ȼ�С�����ڴ���H2S S(����) S2O32����2H��=S����SO2����H2O -lg![]() ȡ���һ��ϴ��Һ�����������ữ����������Һ����������ϴ�Ӹɾ� 2
ȡ���һ��ϴ��Һ�����������ữ����������Һ����������ϴ�Ӹɾ� 2
��������
�����������������Mg2+��Fe3+�ȣ���Ӧ����BaCl2xH2O������������Mg2+��Fe3+�ȣ������ᷴӦ�����������壬�����Ȼ�����Һ�к����H2S��HS-�ȣ�Ӱ���Ʒ�������ɹ���Ԥ�Ⱥ�Ŀ������������ⱻ�����������ɳ���AΪ�����������Һ������ҺPH��ȥMg2+��Fe3+���õ�����BΪMg��OH��2��Fe��OH��3���õ��Ȼ�����Һ����Ũ���õ��Ȼ������壬���еõ���ĸҺѭ��ʹ�ã��ݴ˷�������
��1����ӦI�����ɵ�H2S��������ˮ���գ�������泥�����������������Ӧ���ɵõ�������ʹ����Һ������˵���������˰�ˮ��������Ӧ�Ļ�ѧ����ʽΪ2(NH4)2S ��O2��2H2O = 4NH3��H2O��2S����
��2�������Ȼ�����Һ�к�����(H2S��HS����)Ӱ���Ʒ�������ɹ���Ԥ�Ⱥ�Ŀ���������Ԥ�ȿ�����Ŀ�������½�ʹ������ܽ�ȼ�С�������ڴ���H2S�������ȿ����Ĺ������̳�Ϊ�������Գ���A����Ҫ�ɷ���S��
��3��S2O32����������Һ�лᷢ�������绯��Ӧ������S��SO2�������ữ����ʱ�����ӷ���ʽΪS2O32����2H��=S����SO2����H2O��
��4������Ksp[Mg(OH)2]=1.8��10��11��c(OH��)2��10��5=1.8��10��11�����c(OH��)=![]() ��10��3mol/L������ Ksp[Fe(OH)3]=4.0��10��38��c(OH��)3��10��5=4.0��10��32�����c(OH��)=
��10��3mol/L������ Ksp[Fe(OH)3]=4.0��10��38��c(OH��)3��10��5=4.0��10��32�����c(OH��)=![]() ��10��9mol/L��˵��Mg(OH)2�ܽ�Ƚϴ�Mg(OH)2��ȫ�γɳ�������pH����pH=-lg
��10��9mol/L��˵��Mg(OH)2�ܽ�Ƚϴ�Mg(OH)2��ȫ�γɳ�������pH����pH=-lg![]() ��
��
��5�����ᱵ�������������г�������������BaSO4�����Ƿ�ϴ�Ӹɾ������Լ���ϴ��Һ���Ƿ���Cl�����ɣ����巽����ȡ���һ��ϴ��Һ�����������ữ����������Һ����������ϴ�Ӹɾ�������Ba2���غ�ɵù�ϵʽΪ
BaCl2��xH2O����BaSO4
208��18x 233
12.23 11.65
��� (208��18x):233=12.23 : 11.65 ���x=2��

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�����Ŀ�����ʵ���У���Ӧ������ͽ��۶���ȷ�������߾��������ϵ���ǣ� ��
ѡ�� | ʵ�� | ���� | ���� |
A | ������̼��Ʒ�ĩ���뵽����NH4Cl��Һ�� | �������壬��ĩ�ܽ� | NH4Clˮ��ʹ��Һ������ |
B | ��BaSO4�����ĩ���뱥��Na2CO3��Һ�У����裬���ˣ�ϴ�ӣ��������м���ϡ���� | �������壬���������ܽ� | Ksp(BaCO3)<Ksp(BaSO4) |
C | ����ɫ����ͭ��ĩ���и��¼��� | ��ɫ��ɺ�ɫ | CuO�ֽ�����ͭ���� |
D | ��ij��Һ�μӹ�����ϡ���� | �д̼�����ζ�����������Һ�г��ֳ��� | ��Һ��һ������S2-��SO32- |
A.AB.BC.CD.D