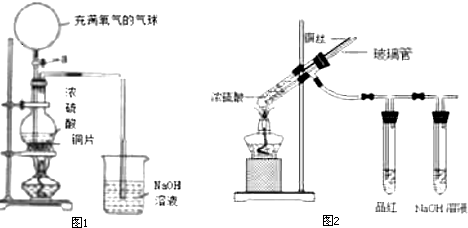
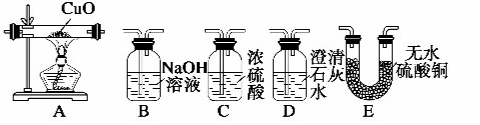
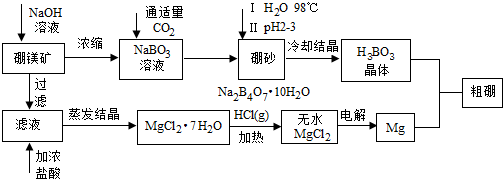
��Ŀ����
16��A��B��C��D��E��FΪԪ�����ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������A��B��C��D��E��F��˳������A��ԭ�Ӱ뾶��С��ԭ�ӣ�B�������������Ǵ�����������2����D��F������������ȣ�F������������к���60%��E��D���γ�E2D��E2D2�������ӻ������д���пհף���1��A��ԭ�ӽṹʾ��ͼ
 ��C�����ڱ��е�λ���ǵڶ����ڵڢ�A�壬�ӽṹ�Ƕ��ж�A2Ӧ��C2���� �����ȶ�����ã������ǵ��������ļ��ܴ���H-H
��C�����ڱ��е�λ���ǵڶ����ڵڢ�A�壬�ӽṹ�Ƕ��ж�A2Ӧ��C2���� �����ȶ�����ã������ǵ��������ļ��ܴ���H-H��2��д��A2F�ĽṹʽH-S-H��E2D2�ĵ���ʽ
 �����к��еĻ�ѧ�����������Ӽ����ۼ�
�����к��еĻ�ѧ�����������Ӽ����ۼ���3���õ���ʽ��ʾBD2��������γɹ���
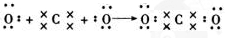 ��
��
���� A��B��C��D��E��FΪԪ�����ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������A��B��C��D��E��F��˳������A��ԭ�Ӱ뾶��С��ԭ�ӣ���AΪHԪ�أ�B�������������Ǵ�����������2�����������ຬ��8�����ӣ���A������㺬��4�����ӣ�ΪCԪ�أ�E��D���γ�E2D��E2D2�������ӻ������EΪNa��DΪOԪ�أ�D��F������������ȣ�����λ��ͬһ���壬��FΪSԪ�أ�F�����������ΪSO3�����������к���60%������������C��ԭ����������C��O֮�䣬��CΪNԪ�أ��ݴ˽��н��
��� �⣺A��B��C��D��E��FΪԪ�����ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������A��B��C��D��E��F��˳������A��ԭ�Ӱ뾶��С��ԭ�ӣ���AΪHԪ�أ�B�������������Ǵ�����������2�����������ຬ��8�����ӣ���A������㺬��4�����ӣ�ΪCԪ�أ�E��D���γ�E2D��E2D2�������ӻ������EΪNa��DΪOԪ�أ�D��F������������ȣ�����λ��ͬһ���壬��FΪSԪ�أ�F�����������ΪSO3�����������к���60%������������C��ԭ����������C��O֮�䣬��CΪNԪ�أ�
��1��AΪHԪ�أ�ԭ������Ϊ1����ԭ�ӽṹʾ��ͼΪ��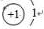 ��CΪNԪ�أ�NԪ�صĺ˵����Ϊ7������㺬��5�����ӣ���Ԫ��λ�����ڱ��еڶ����ڵڢ�A�壻H2�����д������ⵥ����N2�����д��ڵ������������������ļ��ܴ���H-H������Խ��ѧ��Խ���ѣ����ӵĻ�ѧ����Խ�ȶ������Ե����������ȶ���
��CΪNԪ�أ�NԪ�صĺ˵����Ϊ7������㺬��5�����ӣ���Ԫ��λ�����ڱ��еڶ����ڵڢ�A�壻H2�����д������ⵥ����N2�����д��ڵ������������������ļ��ܴ���H-H������Խ��ѧ��Խ���ѣ����ӵĻ�ѧ����Խ�ȶ������Ե����������ȶ���
�ʴ�Ϊ��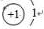 ���ڶ����ڵڢ�A�壻���ã����������ļ��ܴ���H-H��
���ڶ����ڵڢ�A�壻���ã����������ļ��ܴ���H-H��
��2��A2FΪH2S��H2S��������2�Թ��õ��Ӷԣ���H2S�ṹʽH-S-H��D2C2ΪNa2O2����������Ϊ���ӻ���������ʽΪ�� �����������к������Ӽ����ۼ���
�����������к������Ӽ����ۼ���
�ʴ�Ϊ��H-S-H�� �����Ӽ����ۼ���
�����Ӽ����ۼ���
��3��BD2ΪCO2��CO2Ϊ���ۻ�����õ���ʽ��ʾCO2��������γɹ���Ϊ��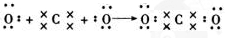 ��
��
�ʴ�Ϊ��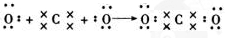 ��
��
���� ���⿼����λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ��ƶϸ�Ԫ��Ϊ���ؼ���ע�����ճ�����ѧ����ĸ����дԭ����ȷԪ�����������ݡ�Ԫ�����ڱ��ṹ��

 һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д� W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ14��������Ϊ7��X��������NH4+������ͬ�����ӡ�������Ŀ��W��Y����������ܵ���������γɣ�Z�ķǽ�������ͬ��������Ԫ������ǿ��
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ14��������Ϊ7��X��������NH4+������ͬ�����ӡ�������Ŀ��W��Y����������ܵ���������γɣ�Z�ķǽ�������ͬ��������Ԫ������ǿ����1��Y�����ڱ��е�λ���ǵ������ڵڢ�A�壮
��2������һ��������NH4+������ͬ�����ӡ�������Ŀ�������ӵĻ�ѧʽΪH3O+��
��3��X3W��ˮ���ͷų�ʹ��̪��Һ��������A����ѧ����ʽ��Na3N+3H2O=NH3��+3NaOH��
��4����֪W�ĵ���������B��һ�������¿��γ�����A������
W2��g��+3B��g��?2A��g����H=-92.4 kJ?mol-1
��ij�¶�ʱ��һ���ݻ��̶����ܱ������У�����������Ӧ���ڲ�ͬʱ��ⶨ�������ڸ����ʵ�Ũ�����±���
| ʱ�� | Ũ�ȣ�mol/L�� | ||
| c��W2�� | c��B�� | c��A�� | |
| ��0 min | 4.0 | 9.0 | 0 |
| ��10min | 3.8 | 8.4 | 0.4 |
| ��20min | 3.4 | 7.2 | 1.2 |
| ��30min | 3.4 | 7.2 | 1.2 |
| ��40min | 3.6 | 7.8 | 0.8 |
�ڷ�Ӧ�ڵ�10min�ı��˷�Ӧ�������ı������������ab��
a�������˴��� b�������¶� c������ѹǿ d������B��Ũ�ȣ�
��Ʒ����Һ��������KMnO4������ˮ���ܵ��з�̪��NaOH���ݺ�I2�ĵ�����Һ��
| A�� | �٢� | B�� | �٢ڢ� | C�� | �ڢۢ� | D�� | �ۢ� |
| A�� | ����ͬλ���У�Tiԭ�Ӻ��е�������������Ϊ30 | |
| B�� | �����а���5����ԭ�ӣ�����Ԫ�ص�5�ֺ��� | |
| C�� | ��������������Ԫ�ص�ƽ�����ԭ������Ϊ48 | |
| D�� | ��Ԫ�������ɽ���Ԫ�� |
�ٱ�����ʹ���Ը��������Һ��ɫ��
�ڱ�������̼̼���ļ�������ȣ�
�۱����ڼ��Ⱥ��д������ڵ���������H2�����ӳɷ�Ӧ���ɻ����飻
�ܾ�ʵ�����ڶ��ױ�����һ�ֽṹ��
�ݱ������廯�����ڵ���������Һ�巢��ȡ����Ӧ��������ѧ�仯��ʹ��ˮ��ɫ��
| A�� | �ڢۢܢ� | B�� | �٢ۢܢ� | C�� | �٢ڢܢ� | D�� | �٢ڢۢ� |
 ��������֪ʶ�ж�����˵���в���ȷ���ǣ�������
��������֪ʶ�ж�����˵���в���ȷ���ǣ�������| A�� | ��ʹ���Ը��������Һ��ɫ | B�� | �ɸ�������Һ��Ӧ�������� | ||
| C�� | ��ʹ��ˮ��ɫ | D�� | ����������ղ�����C10H20O |