��Ŀ����
5��2001��6��21�գ����ϵ�֣�ݡ�����������������ʹ�����������÷�����з�ʽ�������µ�����ȼ��--�����Ҵ����ͣ��Ҵ��������ƾ������������ס�С�������Ϊԭ�Ͼ����͡�������Ƴɵģ��Ҵ���һ����ˮ���ټ����������ͺ��γɱ���ȼ���Ҵ����������Ҵ����;��ǰѱ���ȼ���Ҵ������Ͱ�һ�����������γɵij���ȼ�ϣ�����й�֪ʶ���ش��������⣺��1��д���Ҵ�ȼ�յĻ�ѧ����ʽ��C2H5OH+3O2$\stackrel{��ȼ}{��}$2CO2+3H2O��
��2���Ҵ������������Դ��ԭ��ȼ�ճ�֣������������࣬����ȼ�ղ��ﲻ��Ⱦ������
��3���Ҵ�ȼ��ʱ����������㣬����CO���ɣ���������װ�ã���ͼ��ʾ����֤�Ҵ���ȼ�ղ�������CO��CO2��H2O�������壺
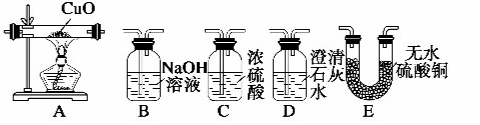
��Ӧ���Ҵ�ȼ�յIJ�������ͨ������дװ�õ���ţ�E��D��B��C��A��������β������������װ��A�з�Ӧ�Ļ�ѧ����ʽ��CO+CuO$\frac{\underline{\;\;��\;\;}}{\;}$Cu+CO2��װ��C�������dz�ȥˮ������װ��E�������Ǽ�������е�ˮ������
�����Ҵ�ȼ�յIJ����У�ˮ��������24.3g����μӷ�Ӧ���Ҵ���������20.7g��
���� ��1���Ҵ��������ڵ�ȼ�������·�Ӧ����ˮ�Ͷ�����̼��
��2�����ݷ�Ӧ���Ⱥ�ȼ�ղ��������
��3������֤�Ҵ���ȼ�ղ�������CO��CO2��H2O��Ӧ������ˮ����ͭ����ˮ���������ó���ʯ��ˮ���������̼�������CuO����CO��
�ڸ�����Ԫ���غ���㣮
��� �⣺��1���Ҵ��������ڵ�ȼ�������·�Ӧ����ˮ�Ͷ�����̼���仯ѧ����ʽΪ��C2H5OH+3O2$\stackrel{��ȼ}{��}$2CO2+3H2O���ʴ�Ϊ��C2H5OH+3O2$\stackrel{��ȼ}{��}$2CO2+3H2O��
��2���Ҵ�ȼ��ʱ�ܳ�ַ�Ӧ�������������࣬����ȼ�ղ���Ϊˮ�Ͷ�����̼����Ⱦ�������ʴ�Ϊ��ȼ�ճ�֣������������࣬����ȼ�ղ��ﲻ��Ⱦ������
��3������֤�Ҵ���ȼ�ղ�������CO��CO2��H2O��Ӧ������ˮ����ͭ����ˮ��������ȼ�յIJ�����ͨ��ʢ����ˮ����ͭ��װ��E�����ó���ʯ��ˮ���������̼������E������������ͨ��D�м��������̼��Ȼ��ͨ��B�г�ȥ����Ķ�����̼���ٽ���C���ù�Ũ�����������A�е�CuO����CO����װ��A����CO��ԭCuO���䷴Ӧ�Ļ�ѧ����ʽΪCO+CuO$\frac{\underline{\;\;��\;\;}}{\;}$Cu+CO2��
�����Ϸ�����֪��װ��C�������dz�ȥˮ������װ��E�������Ǽ�������е�ˮ������
�ʴ�Ϊ��E��D��B��C��A��CO+CuO$\frac{\underline{\;\;��\;\;}}{\;}$Cu+CO2����ȥˮ��������������е�ˮ������
����μӷ�Ӧ���Ҵ�Ϊxg��
C2H5OH������3H2O
46 3��18
xg 24.3g
��x=$\frac{24.3g��46}{3��18}$=20.7g��
�ʴ�Ϊ��20.7g��
���� ���⿼�������ʵļ��顢ʵ�鷽����ơ�����ʽ����д�Լ���ѧ����ȣ���Ŀ�Ѷ��еȣ���Ŀ�漰��֪ʶ��϶࣬�����ڿ���ѧ���Ի���֪ʶ���ۺ�Ӧ��������ʵ��̽��������

Y����4H2O+2O2+8e-=8OH-��
���й��ڴ�ȼ�ϵ�ص�˵���У�������ǣ�������
| A�� | X������Y���� | |
| B�� | �õ�ع���ʱ��X��������Һ�ļ�����ǿ | |
| C�� | �ڱ�״���£�ͨ��5.6LO2��ȫ��Ӧ����1mol���ӷ���ת�� | |
| D�� | ����һ��ʱ���KOH�����ʵ������� |
| A�� | NH3��H2��C4H10? | B�� | PCl3��CO2��H2SO4 | C�� | SO2��SiO2��P2O5? | D�� | CCl4��Na2S��H2O2 |
| A�� | 2.24L CO2�к��е�ԭ����Ϊ0.3NA | B�� | 20g D2O������������Ϊ10NA | ||
| C�� | 16g CH4�к�C-H����ĿΪNA | D�� | 1 mol �������е�ԭ����Ϊ2NA |

��֪�����й��������������pH��
| �������� | Fe��OH��3 | Fe��OH��2 |
| ��ʼ������pH | 1.5 | 6.5 |
| ������ȫ��pH | 3.7 | 9.7 |
��1����������Ҫ�ӿ췴Ӧ���ʣ���ʩ�г�ֽ���ͼ��ȡ��ʵ���������Ũ�ȵȣ�дһ�֣���̼���������ᷴӦ�����ӷ���ʽSrCO3+2H+=Sr2++CO2��+H2O��
��2���ڲ����-�۵Ĺ����У�����Һ��pHֵ��1������B�����õ��Լ�ΪE��
A.1.5 B.3.7 C.9.7 D����ˮ E���������ȷ�ĩ F��̼���ƾ���
��3����������������������Ҫ�ɷ���Fe��OH��3��BaSO4 ���ѧʽ����
��4����ҵ�����ȷ紵����ˮ�Ȼ��ȣ����˵��¶���A
A��50��60��B��80��100��C��100������
��5���������ѡ�õ���ϴ�Ӽ��DZ����Ȼ�����Һ��
| A�� | CaCl2 | B�� | Na2O | C�� | H2SO4 | D�� | NH4Cl |
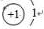 ��C�����ڱ��е�λ���ǵڶ����ڵڢ�A�壬�ӽṹ�Ƕ��ж�A2Ӧ��C2���� �����ȶ�����ã������ǵ��������ļ��ܴ���H-H
��C�����ڱ��е�λ���ǵڶ����ڵڢ�A�壬�ӽṹ�Ƕ��ж�A2Ӧ��C2���� �����ȶ�����ã������ǵ��������ļ��ܴ���H-H �����к��еĻ�ѧ�����������Ӽ����ۼ�
�����к��еĻ�ѧ�����������Ӽ����ۼ�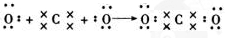 ��
��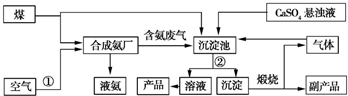
 2NH3��
2NH3�� CaCO3��+��NH4��2SO4��
CaCO3��+��NH4��2SO4��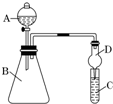 ijͬѧΪ̽��Ԫ�����ڱ���Ԫ�����ʵĵݱ���ɣ����������ϵ��ʵ�飮
ijͬѧΪ̽��Ԫ�����ڱ���Ԫ�����ʵĵݱ���ɣ����������ϵ��ʵ�飮