��Ŀ����
12�� �к�����ָ�������кͷ�Ӧ����l mol H2O���ų���������ijѧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ���50mL0.5mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ����ش��������⣺
�к�����ָ�������кͷ�Ӧ����l mol H2O���ų���������ijѧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ���50mL0.5mol/L��������50mL0.55mol/L��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ����ش��������⣺�ٴ�ʵ��װ�ÿ����ձ���������������ĭ�������DZ��¡����ȣ�����ʵ������е�������ʧ��
�ڴ��ձ����粻��Ӳֽ�壬����õ��к�����ֵƫС���ƫ����ƫС��������Ӱ�족��
��ʵ���и���60mL 0.50mol/L�������50mL 0.55mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ����ȡ�����ȡ����������к��ȵ���ֵ��ȣ����ȡ�����ȡ�����
���� ��1�������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��2������Ӳֽ�壬����һ��������ɢʧ��
��3����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��� �⣺��1���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮��������ֽ���������DZ��¡����ȣ�����ʵ������е�������ʧ��
�ʴ�Ϊ�����¡����ȣ�����ʵ������е�������ʧ��
��2�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С���ʴ�Ϊ��ƫС��
��3����Ӧ�ų����������������Լ�������Ķ����йأ���60mL 0.50mol/L�������50mL 0.55mol/L��NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�����ʵ������ӣ��ų�������ƫ�ߣ����к�����ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�������60mL 0.50mol/L�������50mL 0.55mol/L��NaOH��Һ������ʵ�飬����к�����ֵ��ȣ�
�ʴ�Ϊ������ȣ���ȣ�
���� ���⿼��ѧ���й��к��ȵIJⶨԭ�����������������ע���к������ᡢ������ʵ����أ��ѶȲ���

��ϰ��ϵ�д�
 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
�����Ŀ
19������ʵ�������Ҫ�õ������������в�������������ͬ���Ǣٹ��� ������ ���ܽ� ��Ũ�����ϡ�ͣ�������
| A�� | �ٺ͢� | B�� | �ٺ͢� | C�� | �ں͢� | D�� | �ں͢� |
20��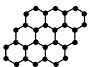 ���ʯ����ṹ�У�ÿ��̼ԭ������Χ�ĸ�̼ԭ���γ���������ռ�������״�ṹ������ʯ������̼ԭ�����빲�ۼ�֮���ǣ�������
���ʯ����ṹ�У�ÿ��̼ԭ������Χ�ĸ�̼ԭ���γ���������ռ�������״�ṹ������ʯ������̼ԭ�����빲�ۼ�֮���ǣ�������
 ���ʯ����ṹ�У�ÿ��̼ԭ������Χ�ĸ�̼ԭ���γ���������ռ�������״�ṹ������ʯ������̼ԭ�����빲�ۼ�֮���ǣ�������
���ʯ����ṹ�У�ÿ��̼ԭ������Χ�ĸ�̼ԭ���γ���������ռ�������״�ṹ������ʯ������̼ԭ�����빲�ۼ�֮���ǣ�������| A�� | 1��2 | B�� | 2��1 | C�� | 1��4 | D�� | 4��1 |
7���˵�����ֱ���16��4��Ԫ��ԭ����Ƚϣ�ǰ�ߵ����������Ǻ���4�����ǣ�������
�������������������������۵��Ӳ������ܵ���������
�������������������������۵��Ӳ������ܵ���������
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ۢ� |
4����ͼΪԪ�����ڱ��ж����ڵ�һ���֣���Xԭ�Ӵ�����������������������3��������˵����ȷ���ǣ�������
| R | ||
| X | Y | Z |
| A�� | X���⻯���R���⻯���ȶ� | |
| B�� | ԭ�Ӱ뾶��С˳����Z��Y��X | |
| C�� | YԪ�����γ����ֳ����ĺ����ᣬ����һ����ǿ�� | |
| D�� | XZ5�����и�ԭ�Ӿ����������8���ӽṹ |
1��������Ƽ��˵��������ǣ�������
| A�� | ��Ƽ��ǹ��ʰ�ί���ϸ��ֹʹ�õ��˷ܼ� | |
| B�� | ��Ƽ��Ǵ���ҩ����ȡ����Ȼҩ�� | |
| C�� | ��Ƽ���ʹ���˷ܣ��˶�Ա���ú��ܳ�ˮƽ���� | |
| D�� | ��Ƽ������ߵ�Ч�� |
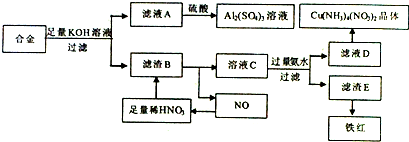

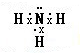 �����Ļ�ѧ�����ڼ��Լ����������Ӽ����ۼ��Ļ�����Ļ�ѧʽΪNa2O2��
�����Ļ�ѧ�����ڼ��Լ����������Ӽ����ۼ��Ļ�����Ļ�ѧʽΪNa2O2�� ��
��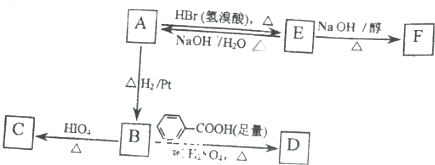
 $��_{��}^{HIO_{4}}$RCHO+R��CHO
$��_{��}^{HIO_{4}}$RCHO+R��CHO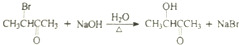 ��
��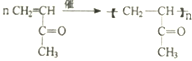 ��
��