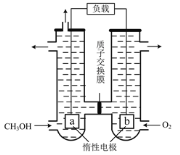
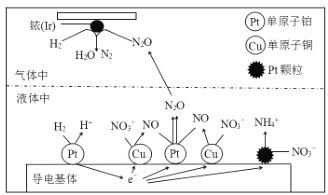
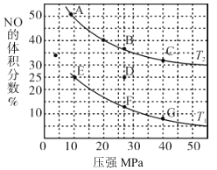
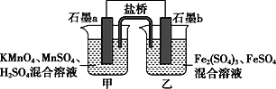
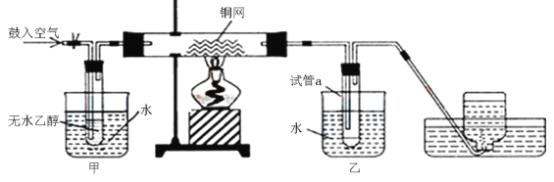
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΜ·ΚœΈοM «÷Τ±Η“Μ÷÷ΩΙΨζ“©ΒΡ÷–ΦδΧεΘ§ Β―ι ““‘ΖΦœψΜ·ΚœΈοAΈΣ‘≠Νœ÷Τ±ΗMΒΡ“Μ÷÷Κœ≥…¬ΖœΏ»γœ¬ΘΚ
“―÷ΣΘΚR1CH2Br![]() R1CH=CHR2
R1CH=CHR2![]()
![]()
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)BΒΡΜ·―ßΟϊ≥ΤΈΣ_________ΘΜE÷–ΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣ__________ ΓΘ
(2)CΓζDΒΡΖ¥”Πάύ–ΆΈΣ_______ΓΘ
(3)–¥≥ωD”κ«β―θΜ·ΡΤΥ°»ή“ΚΙ≤»»ΒΡΜ·―ßΖΫ≥Χ Ϋ________________________ΓΘ
(4)”…F…ζ≥…MΥυ–ηΒΡ ‘ΦΝΚΆΧθΦΰΈΣ_____________ ΓΘ
(5)X «DΒΡΆ§Ζ÷“λΙΙΧεΘ§Ά§ ±ΖϊΚœœ¬Ν–ΧθΦΰΒΡXΩ…ΡήΒΡΫαΙΙ”–______÷÷(≤ΜΚ§ΝΔΧε“λΙΙ)ΓΘ
ΔΌ±ΫΜΖ…œ”–ΝΫΗω»Γ¥ζΜυΘ§Κ§ΝΫΗωΙΌΡήΆ≈ΘΜ ΔΎΡήΖΔ…ζ“χΨΒΖ¥”ΠΓΘ
Τδ÷–ΚΥ¥≈Ι≤’ώ«βΤΉœ‘ Ψ4ΉιΖεΒΡΫαΙΙΦρ Ϋ «___________(»Έ–¥“Μ÷÷)ΓΘ
(6)ΧΦ‘≠Ή”…œΝ§”–4Ηω≤ΜΆ§ΒΡ‘≠Ή”Μρ‘≠Ή”Ά≈ ±Θ§ΗΟΧΦ≥ΤΈΣ ÷–‘ΧΦΓΘ–¥≥ωFΒΡΫαΙΙΦρ Ϋ____________Θ§”Ο–«Κ≈(*)±ξ≥ωF÷–ΒΡ ÷–‘ΧΦΓΘ
(7)≤Έ’’…œ ωΚœ≥…¬ΖœΏΚΆ–≈œΔΘ§“‘““œ©ΚΆ““»©ΈΣ‘≠Νœ(ΈόΜζ ‘ΦΝ»Έ―Γ)Θ§…ηΦΤ÷Τ±Η![]() ΒΡΚœ≥…¬ΖœΏΓΘ______________________________ΓΘ
ΒΡΚœ≥…¬ΖœΏΓΘ______________________________ΓΘ
ΓΨ¥πΑΗΓΩΝΎΦΉΜυ±ΫΦΉ»©Μρ2-ΦΉΜυ±ΫΦΉ»© ΧΦΧΦΥΪΦϋΓΔτ»Μυ »Γ¥ζΖ¥”Π 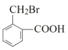 +2NaOH
+2NaOH![]()
![]() +NaBr+H2O NaOHΒΡ““¥Φ»ή“ΚΓΔΦ”»» 6
+NaBr+H2O NaOHΒΡ““¥Φ»ή“ΚΓΔΦ”»» 6 ![]() ΓΔ
ΓΔ![]()
![]()
ΓΨΫβΈωΓΩ
AΖ÷Ή” Ϋ «C7H8Θ§‘ρA «ΦΉ±ΫΘ§ΫαΙΙΦρ Ϋ «![]() Θ§A”κCO‘ΎAlCl3/HCl¥φ‘ΎΧθΦΰœ¬ΖΔ…ζΖ¥”Π…ζ≥…BΘ§BΖ÷Ή” Ϋ «C8H8OΘ§B”κO2Ζ¥”Π…ζ≥…ΒΡC «
Θ§A”κCO‘ΎAlCl3/HCl¥φ‘ΎΧθΦΰœ¬ΖΔ…ζΖ¥”Π…ζ≥…BΘ§BΖ÷Ή” Ϋ «C8H8OΘ§B”κO2Ζ¥”Π…ζ≥…ΒΡC «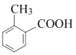 Θ§CΖ÷Ή” Ϋ «C8H8O2Θ§ΝΫ÷÷Έο÷ Ζ÷Ή” Ϋœύ≤ν“ΜΗωO‘≠Ή”Θ§Ω…ΡφΆΤB «
Θ§CΖ÷Ή” Ϋ «C8H8O2Θ§ΝΫ÷÷Έο÷ Ζ÷Ή” Ϋœύ≤ν“ΜΗωO‘≠Ή”Θ§Ω…ΡφΆΤB «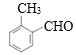 Θ§CΖΔ…ζΦΉΜυ…œΒΡ»Γ¥ζΖ¥”Π…ζ≥…ΒΡDΈΣ
Θ§CΖΔ…ζΦΉΜυ…œΒΡ»Γ¥ζΖ¥”Π…ζ≥…ΒΡDΈΣ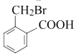 Θ§ΗυΨί“―÷Σ Θ§D”κHCHO‘Ύ“ΜΕ®ΧθΦΰœ¬ΖΔ…ζΖ¥”Π…ζ≥…ΒΡEΈΣΘΚ
Θ§ΗυΨί“―÷Σ Θ§D”κHCHO‘Ύ“ΜΕ®ΧθΦΰœ¬ΖΔ…ζΖ¥”Π…ζ≥…ΒΡEΈΣΘΚ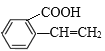 Θ§E”κI2‘Ύ“ΜΕ®ΧθΦΰœ¬ΖΔ…ζ–≈œΔ÷–ΒΎΕΰ≤ΫΖ¥”Π…ζ≥…ΒΡFΈΣ
Θ§E”κI2‘Ύ“ΜΕ®ΧθΦΰœ¬ΖΔ…ζ–≈œΔ÷–ΒΎΕΰ≤ΫΖ¥”Π…ζ≥…ΒΡFΈΣ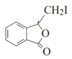 Θ§F”κNaOHΒΡ““¥Φ»ή“ΚΙ≤»»Θ§ΖΔ…ζœϊ»ΞΖ¥”Π≤ζ…ζΒΡMΈΣ
Θ§F”κNaOHΒΡ““¥Φ»ή“ΚΙ≤»»Θ§ΖΔ…ζœϊ»ΞΖ¥”Π≤ζ…ζΒΡMΈΣ![]() ΓΘ
ΓΘ
ΗυΨί…œ ωΖ÷ΈωΩ…÷ΣΘΚA «![]() Θ§B «
Θ§B « Θ§E «
Θ§E « Θ§F «
Θ§F « ΓΘ
ΓΘ
(1)BΫαΙΙΦρ Ϋ « Θ§Οϊ≥ΤΈΣΝΎΦΉΜυ±ΫΦΉ»©Μρ2-ΦΉΜυ±ΫΦΉ»©ΘΜE «
Θ§Οϊ≥ΤΈΣΝΎΦΉΜυ±ΫΦΉ»©Μρ2-ΦΉΜυ±ΫΦΉ»©ΘΜE « Θ§Κ§”–ΒΡΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣΧΦΧΦΥΪΦϋΓΔτ»ΜυΘΜ
Θ§Κ§”–ΒΡΙΌΡήΆ≈ΒΡΟϊ≥ΤΈΣΧΦΧΦΥΪΦϋΓΔτ»ΜυΘΜ
(2) C « Θ§D «
Θ§D « Θ§ «ΦΉΜυ…œΒΡ“ΜΗωH‘≠Ή”±ΜBr‘≠Ή”»Γ¥ζΘ§Ι CΓζDΒΡΖ¥”Πάύ–ΆΈΣ»Γ¥ζΖ¥”ΠΘΜ
Θ§ «ΦΉΜυ…œΒΡ“ΜΗωH‘≠Ή”±ΜBr‘≠Ή”»Γ¥ζΘ§Ι CΓζDΒΡΖ¥”Πάύ–ΆΈΣ»Γ¥ζΖ¥”ΠΘΜ
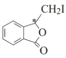
(3) D « Θ§D”κ«β―θΜ·ΡΤΥ°»ή“ΚΙ≤»»Θ§Br‘≠Ή”±Μ-OH»Γ¥ζΘ§-COOH”κNaOHΖΔ…ζ÷–ΚΆΖ¥”Π≤ζ…ζ-COONaΘ§Ι Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ
Θ§D”κ«β―θΜ·ΡΤΥ°»ή“ΚΙ≤»»Θ§Br‘≠Ή”±Μ-OH»Γ¥ζΘ§-COOH”κNaOHΖΔ…ζ÷–ΚΆΖ¥”Π≤ζ…ζ-COONaΘ§Ι Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ +2NaOH
+2NaOH![]()
![]() +NaBr+H2OΘΜ
+NaBr+H2OΘΜ
(4) F « Θ§Ζ÷Ή”÷–Κ§”–I‘≠Ή”Θ§”…”ΎI‘≠Ή”Ν§Ϋ”ΒΡC‘≠Ή”ΒΡΝΎΈΜC‘≠Ή”…œΚ§”–H‘≠Ή”Θ§Υυ“‘FΩ…“‘”κNaOHΒΡ““¥Φ»ή“Κ‘ΎΦ”»»ΧθΦΰœ¬ΖΔ…ζœϊ»ΞΖ¥”Π–Έ≥…≤Μ±ΞΚΆΒΡΧΦΧΦΥΪΦϋΘ§ΒΟΒΫΈο÷ MΘΚ
Θ§Ζ÷Ή”÷–Κ§”–I‘≠Ή”Θ§”…”ΎI‘≠Ή”Ν§Ϋ”ΒΡC‘≠Ή”ΒΡΝΎΈΜC‘≠Ή”…œΚ§”–H‘≠Ή”Θ§Υυ“‘FΩ…“‘”κNaOHΒΡ““¥Φ»ή“Κ‘ΎΦ”»»ΧθΦΰœ¬ΖΔ…ζœϊ»ΞΖ¥”Π–Έ≥…≤Μ±ΞΚΆΒΡΧΦΧΦΥΪΦϋΘ§ΒΟΒΫΈο÷ MΘΚ![]() Θ§Ι ”…F…ζ≥…MΥυ–ηΒΡ ‘ΦΝΚΆΧθΦΰΈΣNaOHΒΡ““¥Φ»ή“ΚΓΔΦ”»»ΘΜ
Θ§Ι ”…F…ζ≥…MΥυ–ηΒΡ ‘ΦΝΚΆΧθΦΰΈΣNaOHΒΡ““¥Φ»ή“ΚΓΔΦ”»»ΘΜ
(5)D « Θ§X «DΒΡΆ§Ζ÷“λΙΙΧεΘ§Ά§ ±ΖϊΚœœ¬Ν–ΧθΦΰΘΚΔΌ±ΫΜΖ…œ”–ΝΫΗω»Γ¥ζΜυΘ§Κ§ΝΫΗωΙΌΡήΆ≈ΘΜ ΔΎΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§ΥΒΟςΚ§”–»©Μυ-CHOΜρΦΉΥα–Έ≥…ΒΡθΞΜυHCOO-Θ§”…”ΎX÷–Κ§”–2ΗωO‘≠Ή”Θ§Ω…»ΖΕ®XΩ…ΡήΚ§”–ΝΫΗω»Γ¥ζΜυΖ÷±π «HCOO-ΓΔ-CH2BrΜρHCOOCH2-ΓΔ-BrΘ§ΝΫΗωΙΌΡήΆ≈‘Ύ±ΫΜΖ…œΒΡΈΜ÷Ο”–ΝΎΈΜΓΔΦδΈΜΓΔΕ‘ΈΜ»ΐ÷÷≤ΜΆ§ΒΡΈΜ÷ΟΘ§“ρ¥ΥDΒΡΆ§Ζ÷“λΙΙΧεXΩ…ΡήΒΡ÷÷άύ ΐΡΩΈΣ2ΓΝ3=6÷÷ΘΜΤδ÷–ΚΥ¥≈Ι≤’ώ«βΤΉœ‘ Ψ4ΉιΖεΒΡΫαΙΙΦρ Ϋ «
Θ§X «DΒΡΆ§Ζ÷“λΙΙΧεΘ§Ά§ ±ΖϊΚœœ¬Ν–ΧθΦΰΘΚΔΌ±ΫΜΖ…œ”–ΝΫΗω»Γ¥ζΜυΘ§Κ§ΝΫΗωΙΌΡήΆ≈ΘΜ ΔΎΡήΖΔ…ζ“χΨΒΖ¥”ΠΘ§ΥΒΟςΚ§”–»©Μυ-CHOΜρΦΉΥα–Έ≥…ΒΡθΞΜυHCOO-Θ§”…”ΎX÷–Κ§”–2ΗωO‘≠Ή”Θ§Ω…»ΖΕ®XΩ…ΡήΚ§”–ΝΫΗω»Γ¥ζΜυΖ÷±π «HCOO-ΓΔ-CH2BrΜρHCOOCH2-ΓΔ-BrΘ§ΝΫΗωΙΌΡήΆ≈‘Ύ±ΫΜΖ…œΒΡΈΜ÷Ο”–ΝΎΈΜΓΔΦδΈΜΓΔΕ‘ΈΜ»ΐ÷÷≤ΜΆ§ΒΡΈΜ÷ΟΘ§“ρ¥ΥDΒΡΆ§Ζ÷“λΙΙΧεXΩ…ΡήΒΡ÷÷άύ ΐΡΩΈΣ2ΓΝ3=6÷÷ΘΜΤδ÷–ΚΥ¥≈Ι≤’ώ«βΤΉœ‘ Ψ4ΉιΖεΒΡΫαΙΙΦρ Ϋ «![]() Μρ
Μρ![]() ΘΜ
ΘΜ
(6)ΧΦ‘≠Ή”…œΝ§”–4Ηω≤ΜΆ§ΒΡ‘≠Ή”Μρ‘≠Ή”Ά≈ ±Θ§ΗΟΧΦ≥ΤΈΣ ÷–‘ΧΦΘΜFΒΡΫαΙΙΦρ Ϋ « Θ§Τδ÷–Κ§”–ΒΡ ÷–‘ΧΦ‘≠Ή””Ο–«Κ≈(*)±ξ≥ωΈΣΘΚ
Θ§Τδ÷–Κ§”–ΒΡ ÷–‘ΧΦ‘≠Ή””Ο–«Κ≈(*)±ξ≥ωΈΣΘΚ ΘΜ
ΘΜ
(7)CH2=CH2”κHBr‘Ύ¥ΏΜ·ΦΝ¥φ‘ΎΧθΦΰœ¬ΖΔ…ζΦ”≥…Ζ¥”Π≤ζ…ζCH3CH2BrΘ§ΗυΨίΧβΗχ“―÷ΣΘ§CH3CH2Br”κCH3CHOΖ¥”Π≤ζ…ζCH3CH=CHCH3Θ§CH3CH=CHCH3‘Ύ¥ΏΜ·ΦΝ¥φ‘ΎΧθΦΰœ¬Φ”»»Θ§ΖΔ…ζΦ”ΨέΖ¥”Π≤ζ…ζ![]() Θ§Ι “‘““œ©ΚΆ““»©ΈΣ‘≠Νœ÷Τ±Η
Θ§Ι “‘““œ©ΚΆ““»©ΈΣ‘≠Νœ÷Τ±Η![]() ΒΡΚœ≥…¬ΖœΏΈΣΘΚ
ΒΡΚœ≥…¬ΖœΏΈΣΘΚ![]() ΓΘ
ΓΘ

 ΉΏΫχΈΡ―‘ΈΡœΒΝ–¥πΑΗ
ΉΏΫχΈΡ―‘ΈΡœΒΝ–¥πΑΗ