��Ŀ����
12��ijͬѧ����ϡ������п��ȡ������ʵ���У����ּ�����������ͭ��Һ�ɼӿ��������������ʣ���ش��������⣺��1������ͭ��Һ���Լӿ������������ʵ�ԭ����CuSO4��Zn��Ӧ������Cu��Zn�γ�Cu-Znԭ��أ��ӿ����������ɵ�����
��2��ʵ����������Na2SO4��MgSO4��Ag2SO4��K2SO4��4����Һ����������ʵ����CuSO4��Һ���������õ���Ag2SO4��
��3��Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬��ͬѧ���������һϵ��ʵ�飮�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䣮
| ʵ�� �����Һ | A | B | C | D | E | F |
| 4mol/L H2SO 4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O/mL | V7 | V8 | V9 | V10 | 10 | 0 |
�ڸ�ͬѧ���ó��Ľ���Ϊ������������CuSO4��Һʱ���������������ʻ�����ߣ����������CuSO4��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ���ǵ������CuSO4��Һ����һ����ʱ�����ɵĵ���Cu�������Zn�ı��棬������Zn����Һ�ĽӴ������
���� ��1��ZnΪ���ý��������û�������ͭ�е�Cu����������Һ��ͬ����ԭ��أ�
��2������������Һ�У�Znֻ���û���Ag��
��3����Ҫ�Ա�����Ч������ô���˷�Ӧ�����ʵ�����һ�����⣬Ҫ��֤����������ͬ��������̽������ͭ����Ӱ�죬��ôÿ���������Ҫ������ͬ�����鷴Ӧ�������ҲӦ����ͬ��
������п�û�ͭ���������Ⱥ�˳���Լ����ɵ�ͭ�ḽ����п�����ϻش�
��� �⣺��1��Zn������ķ�Ӧ�м���������CuSO4��Һ�����û���һ����Cu������Һ���γ�Cu-Znԭ��أ��Ӷ��ӿ컯ѧ��Ӧ���ʣ��ʴ�Ϊ��CuSO4��Zn��Ӧ������Cu��Zn�γ�Cu-Znԭ��أ��ӿ����������ɵ����ʣ�
��2������������Һ��ֻZnֻ���û���Ag����Ag2SO4��CuSO4��Һ�������Ƶ����ã��ʴ�Ϊ��Ag2SO4��
��3����Ҫ�Ա�����Ч������ô���˷�Ӧ�����ʵ�����һ�����⣬Ҫ��֤����������ͬ��������̽������ͭ����Ӱ�죬��ôÿ���������Ҫ������ͬ�����鷴Ӧ�������ҲӦ����ͬ��A��������Ϊ30ml����ô������������Ҳ��Ϊ30ml��������ͭ��Һ��ˮ������Ӧ��ͬ��F��������ͭ20ml��ˮΪ0��V1=V5=30mL����ô����Ϊ50ml������V1=30ml��V6=10ml��V7=20ml��V9=50ml-30mL-2.5mL=17.5mL���ʴ�Ϊ��30��10��17.5��
����Ϊп����������ͭ��Ӧ��ֱ������ͭ��Ӧ��������ᷴӦ��������������ͭ���϶�ʱ����Ӧʱ��ϳ����������ɵ�ͭ�ḽ����пƬ�ϣ����谭пƬ�����������Ӧ���������������½����ʴ�Ϊ���������CuSO4��Һ����һ����ʱ�����ɵĵ���Cu�������Zn�ı��棬������Zn����Һ�ĽӴ������
���� ������Ҫ����Ӱ�컯ѧ��Ӧ���ʵ����أ���������ݷ���CuSO4��Һ�Ի�ѧ��Ӧ���ʵ�Ӱ��ȣ����ؿ��鿼��������ѧ֪ʶ������𣬸���ʵ����������˼ά��������Ŀ�ѶȲ���

 ���ݼ���ϵ�д�
���ݼ���ϵ�д�| A�� | ����ʱ��̫�� | B�� | ��Ӧ��ȴ���ٵ���AgNO3��Һ | ||
| C�� | ��AgNO3��Һǰδ��ϡHNO3�ữ | D�� | ��AgNO3��Һ��δ��ϡHNO3 |
 ��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̣���ѧ���ļ������γɣ����1mol��ѧ��ʱ�ͷţ������գ�����������֪����P4O6�ķ��ӽṹ����ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�kJ/mol����P-P��198��P-O��360��O=O��498����ӦP4�����ף�+3O2�TP4O6�ķ�Ӧ�ȡ�HΪ��������
��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̣���ѧ���ļ������γɣ����1mol��ѧ��ʱ�ͷţ������գ�����������֪����P4O6�ķ��ӽṹ����ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�kJ/mol����P-P��198��P-O��360��O=O��498����ӦP4�����ף�+3O2�TP4O6�ķ�Ӧ�ȡ�HΪ��������| A�� | -1 638 kJ/mol | B�� | +1 638 kJ/mol | C�� | -126 kJ/mol | D�� | +126 kJ/mol |
| A�� | Br2 | B�� | CO2 | C�� | NaCl | D�� | HCl |

ʵ���У������Ƶõİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ�ã�������������ͭ����Ӧ��Ϻ�ɫ������ͭת��Ϊ��ɫ��ͭ����ͼA��B��CΪ�ס�����С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��
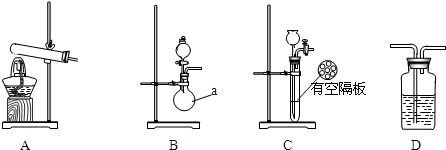
��С���ã���Ӧǰ����ͭ������m1g������ͭ��Ӧ��ʣ����������m2g�����ɵ����ڱ�״���µ����V1L����С���ã�ϴ��ǰװ��D������m3g��ϴ����װ��D������m4g�����ɵ����ڱ�״���µ����V2L��
��ش��������⣺
��1�����Aװ�������ԵIJ��������ӵ��ܣ������ܲ���ˮ�У������Թܣ����ܿ������ݲ�����ֹͣ���ȣ���������ˮ�������γ�һ���ȶ���ˮ����
��2���ס�����С��ѡ���˲�ͬ�ķ�����ȡ�������뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��Ŀո��У�
| ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� | |
| ��С�� | A | �������ơ������ | ��Ӧ�Ļ�ѧ����ʽΪ�� ��NH4��2SO4+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$2NH3��+2H2O+CaSO4�� |
| ��С�� | ��B | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã� �������������ڰ�ˮ����ȣ�����������Ũ�ȣ�ʹNH3+H2O?NH3•H2O?NH4++OH-���淴Ӧ�����ƶ����ӿ찱���ݳ��� |
��4����С������������ݼ�����������е������ԭ�Ӹ���������С������ֵ��Ϊ�ˣ���С����ԭ��ʵ��Ļ�����������һ��װ��ijҩƷ��ʵ������������ʵ�飮����ʵ��ǰ���ҩƷ�������仯�����ɵ�����������ó��˺�����ʵ��������ҩƷ�������Ǽ�ʯ�ң��������ơ������Ƶȣ���

 ��
�� $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$ +H2O��
+H2O��
 ��
�� ��
�� ��
��