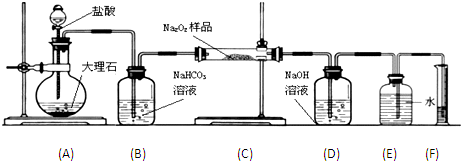
��Ŀ����
17����ͼ1ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺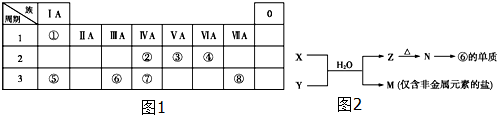
��1��д���ɢܡ��ݡ���Ԫ���γɵļȺ����Ӽ��ֺ����ۼ���һ�����ӻ�����Ļ�ѧʽ��NaClO��
��2����Ԫ�آ�ĵ�����������ˮ�е��л��ﷴӦ���ɶ�������Σ�������ʣ���������̭�����пɴ�����������ˮ����������AC��
A��ClO2 B��AlCl3 C��K2FeO4
��3��W��������ڵ�ͬ����Ԫ�أ����±���д��H2WO3��Ӧ���ʵĻ�ѧ����ʽ��
| ��� | ���� | ��ѧ����ʽ |
| 1 | ��ԭ�� | H2SO3+Br2+2H2O�TH2SO3+2HBr |
| 2 | ���� | H2SO3+2NaOH=Na2SO3+2H2O |
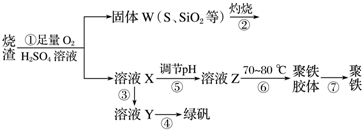
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪAl3++3NH3+3H2O�TAl��OH��3��+3NH4+��
M�������ӵļ���������ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
���M�к���Ԫ�آ࣬M��Һ������Ũ���ɴ�С������˳����c��Cl-����c��NH+4����c��H+����c��OH-����
���� ��Ԫ�������ڱ��е�λ�ÿ�֪����ΪHԪ�ء���ΪCԪ�ء���ΪNԪ�ء���ΪOԪ�ء���ΪNaԪ�ء���ΪAlԪ�ء���ΪSiԪ�ء���ΪSԪ�أ�
��1����O��Na��Cl�γɵļȺ����Ӽ��ֺ����ۼ�������ΪNaClO�ȣ�
��2����Ԫ�آ�ĵ���Ϊ����������ǿ�����ԣ�����ϸ����������ѡ���о���ǿ�����Ե����ʿ������������
��3��W��������ڵ�ͬ����Ԫ�أ���ͼ��֪��������Ԫ�أ���WΪ��Ԫ�أ�H2SO3�������ԡ���ԭ�ԡ������ԣ����巴Ӧ����������HBr��ʾ��ԭ�ԣ����������Ʒ�Ӧ�������ԣ�
��4��M�ǽ����ǽ������Σ�����һ������Σ���ΪAlԪ�أ������ƶ�N��Al2O3��ZΪAl��OH��3����M����ClԪ�أ���XΪAlCl3��YΪNH3��MΪNH4Cl��
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪHԪ�ء���ΪCԪ�ء���ΪNԪ�ء���ΪOԪ�ء���ΪNaԪ�ء���ΪAlԪ�ء���ΪSiԪ�ء���ΪSԪ�أ�
��1����O��Na��Cl�γɵļȺ����Ӽ��ֺ����ۼ�������ΪNaClO�ȣ��ʴ�Ϊ��NaClO��
��2����Ԫ�آ�ĵ���Ϊ����������ǿ�����ԣ�����ϸ����������ѡ���о���ǿ�����Ե����ʿ������������ѡ����ClO2��K2FeO4����ǿ�����ԣ�
�����AC��
��3��W��������ڵ�ͬ����Ԫ�أ���WΪSԪ�أ�H2SO3�ľ��������ԡ���ԭ�ԡ����ԡ����ȶ��Եȣ����Ա�ǿ��������������H2SO3+Br2+2H2O=H2SO3+2HBr����NaOH�����кͷ�ӦH2SO3+2NaOH=Na2SO3+2H2O��
�ʴ�Ϊ��
| 1 | ��ԭ�� | H2SO3+Br2+2H2O�TH2SO3+2HBr |
| 2 | ���� | H2SO3+2NaOH=Na2SO3+2H2O |
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ��Al3++3NH3+3H2O�TAl��OH��3��+3NH4+��
M�������ӵļ�������Ϊ��ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�
���M�к���Ԫ��Cl��ΪNH4Cl��Ϊǿ�������Σ�ˮ������ԣ���Һ������Ũ���ɴ�С������˳���ǣ�c��Cl-����c��NH+4����c��H+����c��OH-����
�ʴ�Ϊ��Al3++3NH3+3H2O�TAl��OH��3��+3NH4+��ȡ����M��Ʒ�����Թܣ���������������Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬֤����笠����ӣ�c��Cl-����c��NH+4����c��H+����c��OH-����
���� ���⿼��Ԫ�����ڱ���������ƶϣ��漰Ԫ�ػ��������ʡ�ʵ�鷽����ơ�����Ũ�ȴ�С�Ƚϵȣ�ע��Ի���֪ʶ�Ļ������գ��Ѷ��еȣ�

| A�� | ���������۵�ܸߣ����Բ���������ұ���� | |
| B�� | ����������һ�ֽ�״�������нϴ��������������ԣ���������ˮ�� | |
| C�� | ʵ���ҿ����������������Ȼ������Ʊ��������� | |
| D�� | ���������ȿ���ǿ�ᷴӦ�ֿ���ǿ�Ӧ���������������� |
| A�� | ����ˮ���������ڻ���� | B�� | �����ܶȱ�ˮ�� | ||
| C�� | �����������ľ����������� | D�� | ����ڱ�����ṹ����ؼ����� |