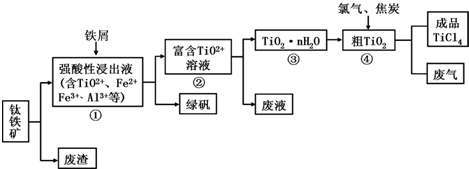
��Ŀ����
3����������ѧ��ѧ�г��������壬����;�㷺��
��1��ʵ������ȡ�����Ļ�ѧ����ʽ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2����ҵ�ϰ��������������͵����ϳ���ͼ1��ʾ��
�ٸ÷�Ӧ���Ȼ�ѧ����ʽ��N2��g��+3H2��g���T2NH3��g����H=-93.36kJ/mol��
�������¶ȵ����ߣ��÷�Ӧ�Ļ�ѧƽ�ⳣ���ı仯�����Ǽ�С��
�ۼ���һ�ּ�鰱���Ƿ�й¶�ɲ��õĻ�ѧ��������ʪ��ĺ�ɫʯ����ֽ�ӽ��������۲���ֽ�Ƿ�������������˵���а���й¶����պȡŨ����ӽ��������۲��Ƿ��а������ɣ�����а�������˵���ܵ�й¶����
��3����ҵ�������β���к��϶��SO2��Ϊ��ֹ��Ⱦ��������������SO2����ҵ�ϳ��ð�ˮ���շ�����β����
�ٵ���ˮ�������������ʵ���Ϊ3mol�����ձ�״����44.8L SO2ʱ����Һ�е�����Ϊ��NH4��2SO3��NH4HSO3��
�ڣ�NH4��2SO3�Լ��ԣ��û�ѧƽ��ԭ������NH4++H2O?NH3•H2O+H+SO32-+H2O?HSO3-+OH-��SO32-ˮ��̶ȴ���NH4+ ��ˮ��̶�ʹ��Һ��c��OH-����c��H+������Һ�ʼ��ԣ�
��4��������һ�ָ���ȼ�ϣ�����ֱ������ȼ�ϵ�أ���ͼ2�ǹ���ˮʽȼ�ϵ�ع���ԭ����
�ٰ���ȼ�ϵ�صĵ������Һ���ѡ����ԣ�����ԡ��������ԡ������ԡ�����Һ��
�ڿ����ڽ�����װ��ǰ��Ҫͨ����������ȥ��������CO2��
�۰���ȼ�ϵ�صķ�Ӧ�ǰ�������������һ�ֳ������������ˮ���õ�صĵ缫�ܷ�Ӧ��4NH3+3O2=2N2+6H2O�������ĵ缫��Ӧʽ��O2+4e-+2H2O=4OH-��
���� ��1��ʵ�������Ȼ�狀��������ƹ��������ȡ������
��2�����������H����H=��Ӧ����ܺ�-��������ܺͼ���д���Ȼ�ѧ����ʽ��
�ڷ�Ӧ�Ƿ��ȷ�Ӧ������ƽ�������ȷ�Ӧ������У�ƽ��������У�
�����ð�����HCl��Ӧ�������̣������Լ��Լ�飻
��3���ٰ������ʵ���Ϊ3mol�����ձ�״����44.8L SO2ʱ��Nԭ��3mol��Sԭ��2mol����ԭ���غ㣬�ڣ�NH4��2SO3�У���ԭ�Ӹ�����N��S=2��1�����У�NH4HSO3����ԭ�Ӹ�����N��S=1��1���ݴ˷�����
�������ε�ˮ�����۽��ͣ�
��4���ٰ����Ǽ������壻
�ڿ����е�CO2�ܺͰ�ˮ��Ӧ��
�����ɵ�������ʱN2���ݴ���д��ѧ����ʽ����������������Ӧ��������������Ӧ��ע��ʱ���Ի�����
��� �⣺��1��ʵ������ȡ�����Ļ�ѧ����ʽ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O���ʴ�Ϊ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2���١�H=��Ӧ����ܺ�-��������ܺ�=945KJ/mol+3��436KJ/mol-6��391.06KJ/mol=-93.36 kJ/mol�����ԣ����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-93.36 kJ/mol���ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-93.36 kJ/mol��
�ڼ����֪��Ӧ�Ƿ��ȷ�Ӧ�����£�ƽ��������У�ƽ�ⳣ����С��
�ʴ�Ϊ����С��
�۰�����HCl��Ӧ�������̣������Լ��ԣ����ԣ���鰱���Ƿ�й¶�ɲ��õĻ�ѧ�����У���ʪ��ĺ�ɫʯ����ֽ�ӽ��������۲���ֽ�Ƿ�������������˵���а���й¶����պȡŨ����ӽ��������۲��Ƿ��а������ɣ�����а�������˵���ܵ�й¶����
�ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ�ӽ��������۲���ֽ�Ƿ�������������˵���а���й¶����պȡŨ����ӽ��������۲��Ƿ��а������ɣ�����а�������˵���ܵ�й¶����
��3���ٰ������ʵ���Ϊ3mol�����ձ�״����44.8L SO2ʱ��Nԭ��3mol��Sԭ��2mol����ԭ���غ㣬�ڣ�NH4��2SO3�У���ԭ�Ӹ�����N��S=2��1�����У�NH4HSO3����ԭ�Ӹ�����N��S=1��1�����ԣ��������У�NH4��2SO3��NH4HSO3���ʴ�Ϊ����NH4��2SO3��NH4HSO3��
�ڣ�NH4��2SO3��Һ�У�笠��������������ˮ�⣬��NH4��2SO3�Լ���˵����NH4++H2O?NH3•H2O+H+SO32-+H2O?HSO3-+OH-��SO32-ˮ��̶ȴ���NH4+ ��ˮ��̶�ʹ��Һ��c��OH-����c��H+������Һ�ʼ��ԣ�
�ʴ�Ϊ��NH4++H2O?NH3•H2O+H+SO32-+H2O?HSO3-+OH-��SO32-ˮ��̶ȴ���NH4+ ��ˮ��̶�ʹ��Һ��c��OH-����c��H+������Һ�ʼ��ԣ�
��4���ٰ����Ǽ������壬���Ե��Һ���ѡ����Եģ��ʴ�Ϊ�����ԣ�
�ڿ����е�CO2�ܺͰ�ˮ��Ӧ�����ԣ���ȥ��������CO2���ʴ�Ϊ��CO2��
�����ɵ�������ʱN2���ݴ���д��ѧ����ʽΪ��4NH3+3O2=2N2+6H2O����������������Ӧ��������������Ӧ��ע��ʱ���Ի��������ԣ������ĵ缫��ӦʽΪ��O2+4e-+2H2O=4OH-���ʴ�Ϊ��4NH3+3O2=2N2+6H2O��O2+4e-+2H2O=4OH-��
���� ���⿼���˵绯ѧ���Ȼ�ѧ����ѧƽ�ⳣ�����㡢�������Һ�Լ����ʼ���ȵȣ����鷶Χ�㣬�Ѷ�һ�㣬��Ҫѧ����ѧϰ������ץס������


��֪������ؽ������������������������pH�����
| �������� | Fe3+ | Fe2+ | Al3+ | Ni2+ |
| ��ʼ������pH | 1.1 | 5.8 | 3.0 | 6.8 |
| ��ȫ������PH | 3.2 | 8.8 | 5.0 | 9.5 |
�۵�ij����Ũ��С��1.0��10-5mol•L-1ʱ����Ϊ��ȫ������
��1����д��һ�������������ʵĴ�ʩ�ѷ�������������ʵ����ȡ��ʵ���������Ũ�ȡ�����ȣ�
��2���Լ�a��һ����ɫ��������д����������ʱ��Ӧ�����ӷ���ʽ2Fe2++H2O2+2H+=2Fe3++2H2O��
��3��pH�ĵ��ط�ΧΪ5.0��pH��6.8��
��4��д����������ʱ��Ӧ�����ӷ���ʽNi2++C2O42-+2H2O=NiC2O4•2H2O����Ca2+������ȫʱ����Һ��c��F-����$\sqrt{\frac{1.46��10{\;}^{-10}}{10{\;}^{-5}}}$mol•L-1��д������ʽ���ɣ���
| A�� | ���Ʋ;߲�������ʢװ�ᡢ��̵�ʳ�� | |
| B�� | ���ڿ������γ�һ�����ܵ������ﱣ��Ĥ�����п���ʴ�ԣ�þ����Ҳ������ | |
| C�� | ����ĥ�������ڿ��������գ������ۻ��������£�˵�����ڿ������Dz�����ȼ�յ� | |
| D�� | �ؿ��к�����ߵĽ������� |
| A�� | ���Ƶ���ˮ��dz����ɫ�����õ���ˮ��ɫ | |
| B�� | ���Ƶ���ˮƯ������ǿ�������õ���ˮƯ�����ú��� | |
| C�� | ���Ƶ���ˮ�����ɷֶ࣬�����õ���ˮ�����ɷ��� | |
| D�� | ���Ƶ���ˮ���������ӣ������õ���ˮ���������� |
 ��ҵ���Ի�����Ϊԭ���������ᣬ������Ҫ��һ���Ǵ������������б��ֺ��º�����������2SO2��g��+O2��g��?2SO3��g����H=-196.6kJ•mol-1
��ҵ���Ի�����Ϊԭ���������ᣬ������Ҫ��һ���Ǵ������������б��ֺ��º�����������2SO2��g��+O2��g��?2SO3��g����H=-196.6kJ•mol-1��1��������Ϊ��߷�Ӧ���ʺ�SO2��ת���ʣ����д�ʩ���е���A��
A����װ���г���O2 B�������¶�C����װ���г���N2 D����װ���г��������SO2
��2��500��ʱ����10mol SO2��5.0mol O2�������Ϊ2L�ĺ����ܱ������У���Ӧ�����������ʾ��
| ʱ��/��min�� | 2 | 4 | 6 | 8 |
| n��SO3��/��mol�� | 4.2 | 8.0 | 9.4 | 9.4 |
��500��ʱ�÷�Ӧ��ƽ�ⳣ��K=1636.3��������1λС������
��3�����º�ѹ��ͨ��3mol SO2��2mol O2�����������ƽ��ʱ�������������Ϊ��ʼʱ��90%������ͬһ��Ӧ�¶ȣ�����ͬ�����У�����ʼ���ʵ�����Ϊ5mol SO2��3.5mol O2��1mol SO3��g��������˵����ȷ����CD��
A����һ��ƽ��ʱ��Ӧ�ų�������Ϊ294.9kJ B������ƽ��SO2��ת�������
C������ƽ��ʱ��O2���������� D���ڶ���ƽ��ʱSO3�������������$\frac{2}{9}$
��4�������500��ʱ��������ʼ�����ͬ���ܱ������г���2mol SO2��1mol O2���������ڷ�Ӧ�����б���ѹǿ���䣬����������������䣬������ά�־��ȣ����������Խ�����ѧƽ�⣮
��ƽ�ⳣ����K ���ף���K������ �����������������=����ͬ�����ﵽƽ��ʱSO2��ת���ʣ������ң�������������
�ڴﵽƽ�������ס����������зֱ�ͨ�������ҵ���Ar���壬�������Ļ�ѧƽ�������ƶ����������Ļ�ѧƽ�ⲻ�ƶ��������������������
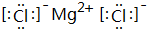 ��
�� ����һ�ֿ����ھ�ˮ����ʳƷ���Σ���A��B��C��D��E���ֶ�����Ԫ����ɣ�������ˮ��ɵ�����������ӣ�����һ������A��B�γɵ�10���������ӣ�AԪ��ԭ�Ӻ�����������E����l��D��Eͬ���壮ijͬѧΪ̽������ɶ���������ʵ�飺
����һ�ֿ����ھ�ˮ����ʳƷ���Σ���A��B��C��D��E���ֶ�����Ԫ����ɣ�������ˮ��ɵ�����������ӣ�����һ������A��B�γɵ�10���������ӣ�AԪ��ԭ�Ӻ�����������E����l��D��Eͬ���壮ijͬѧΪ̽������ɶ���������ʵ�飺