��Ŀ����
10��W��X��Y��Z��M���ֶ�����Ԫ�������ڱ��е�λ����ͼ1��ZY2���γ��������Ҫ����֮һ��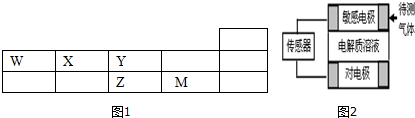
��1��MԪ�ص����ӽṹʾ��ͼΪ

��2��Z��M����Ԫ�ص������̬�⻯����ȶ�����ǿ������˳��ΪHCl��H2S���û�ѧʽ��ʾ����
��3��NaHZˮ��Һ�ʼ��Ե�ԭ����HS-+H2?H2S+OH-���������ӷ���ʽ��ʾ��
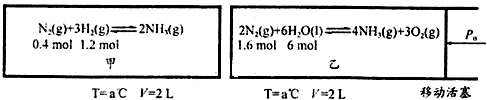
��4��һ��������X2��H2��2L���ܱ������з������·�ӦX2+3H2�T2XH3��H��0������������ݣ�
| ʱ�䣨min�� ���ʵ�����mol�� | 0 | 1 | 2 | 3 | 4 |
| X2 | 3.0 | n1 | 2.4 | n3 | n5 |
| H2 | 9.0 | 8.4 | 7.2 | n4 | n6 |
| XH3 | 0 | 0.4 | n2 | 2.0 | 2.0 |
��5���ڻ���ƽ�����װ��ǿ��ԭ��Һ̬X2H4��ǿ������Һ̬H2Y2�������ǻ��ʱ��ƽ��ÿת��1mol���ӷ���160kJ��ͬʱ����Һ̬ˮ�͵������÷�Ӧ���Ȼ�ѧ����ʽΪN2H4��l��+2H2O2��l��=N2��g��+4H2O��l����H=-640kJ/mol��
��6������������ԭ������ͼ��������ɢ���봫�����������е缫�Ϸ�����Ӧ���������ͻ���յ����źţ�����������WO���������ҺΪH2ZO4���Ե缫����������е���������������˵������ȷ����C��D
A�����е缫���������
B���Ե缫�ϵĵ缫��ӦʽΪ��O2+2H2O+4e-=4OH-
C�����е缫�����������Һ��PH��С
D���������44.8mLWOͨ�����е缫ʱ���������ϵ���ת����ĿΪ0.004NA��
���� W��X��Y��Z��M���ֶ�����Ԫ�������ڱ��е�λ����ͼ1��ZY2���γ��������Ҫ����֮һ���ж�ZΪS��YΪO��MΪCl��WΪC��XΪN��
��1��������Ϊ�ǽ���Ԫ�أ���������������4���õ����ӣ��γ������ӣ���Ԫ����17��Ԫ�أ��������ӵĺ���������Ϊ17�����˵����=�������������������Ϊ�����ӣ�
��2��Z��M����Ԫ�ص������̬�⻯��ΪH2S��HCl�����ݷǽ�����ǿ���ж��軯����ȶ��ԣ�
��3��NaHZˮ��Һ�ʼ��Ե�ԭ����HS-����ˮ�⣬�ƻ���ˮ�ĵ���ƽ������������Ũ��������Һ�Լ��ԣ�
��4�����ͼ�����ݷ�����֪3min��Ӧ�ﵽƽ��״̬�����ݻ�ѧƽ������ʽ��ʽ���㣬ת����=$\frac{������}{��ʼ��}$��100%��ƽ�ⳣ������������ƽ��Ũ���ݴη��˻����Է�Ӧ��ƽ��Ũ�ȵ��ݴη��˻��õ���
��5��ƽ��ÿת��1mol���ӷ���160kJ������Ԫ�ػ��ϼ۱仯��1molN2H4��Ӧ����N2����ת��4mol������4��160KJ=640KJ����ע���ʾۼ�״̬�ͷ�Ӧ�ų���������д�Ȼ�ѧ����ʽ��
��6��A�������屻����������������Ӧ�����е缫Ϊ�����������屻��ԭ�����е缫Ϊ����������������CO����������Ӧ����������
B�����H2S����ʱ������������H2SO4��˵��������Һ�����ԣ������������õ��ӱ���ԭ����ˮ��
C�����е缫Ϊ���������е缫�����缫��Ӧ�������Һ��PH��С
D���������44.8mLCO���ʵ���=$\frac{0.0448L}{22.4L/mol}$=0.02mol��ͨ�����е缫ʱ������Ӧ���ɶ�����̼������ת��0.04mol��
��� �⣺��1��������Ϊ�ǽ���Ԫ�أ���������������4���õ����ӣ��γ������ӣ���Ԫ����17��Ԫ�أ��������ӵĺ���������Ϊ17�����˵����=�������������������Ϊ�����ӣ��������ӵĽṹʾ��ͼ�ǣ� ��
��
�ʴ�Ϊ�� ��
��
��2��Z��M����Ԫ�ص������̬�⻯��ΪH2S��HCl����Ԫ�ػ����Դ�����Ԫ�أ������Ȼ����ȶ��Դ���������ȶ��ԣ�HCl��H2S��
�ʴ�Ϊ��HCl��H2S��
��3��NaHZˮ��Һ�ʼ��Ե�ԭ����HS-����ˮ�⣬�ƻ���ˮ�ĵ���ƽ������������Ũ��������Һ�Լ��ԣ���Ӧ�����ӷ���ʽΪ��HS-+H2O?H2S+OH-��
�ʴ�Ϊ��HS-+H2O?H2S+OH-��
��4�����ݺ���ƽ������ʽ��ʽ���㣬
X2+3H2�T2XH3
��ʼ����mol/L�� 1.5 4.5 0
�仯����mol/L�� 0.5 1.5 1
ƽ������mol/L�� 1 3 1
X2��ת����=$\frac{0.5mol/L}{1.5mol/L}$��100%=33.3%
ƽ�ⳣ��K=$\frac{{1}^{2}}{1��{3}^{3}}$=$\frac{1}{27}$
�ʴ�Ϊ��33.3%��$\frac{1}{27}$��
��5��ƽ��ÿת��1mol���ӷ���160kJ������Ԫ�ػ��ϼ۱仯��1molN2H4��Ӧ����N2����ת��4mol������4��160KJ=640KJ����ע���ʾۼ�״̬�ͷ�Ӧ�ų���������д�Ȼ�ѧ����ʽΪN2H4��l��+2H2O2��l��=N2��g��+4H2O��l����H=-640kJ/mol��
�ʴ�Ϊ��N2H4��l��+2H2O2��l��=N2��g��+4H2O��l����H=-640kJ/mol��
��6��A��ʧ���ӷ���������Ӧ�ĵ缫�Ǹ������õ��ӷ�����ԭ��Ӧ�ĵ缫�����������ݴ�������ͷ�Ӧ�����֪������������CO����������Ӧ������������A����
B�����H2S����ʱ������������H2SO4��˵��������Һ�����ԣ������������õ��ӱ���ԭ����ˮ���缫����ʽΪO2+4H++4e-�T2H2O����B����
C������������CO����������Ӧ�����е缫����������ҺPH��С����C��ȷ��
D���������44.8mLCO���ʵ���=$\frac{0.0448L}{22.4L/mol}$=0.02mol��ͨ�����е缫ʱ������Ӧ���ɶ�����̼������ת��0.04mol������ת����ĿΪ0.004NA����D��ȷ��
�ʴ�Ϊ��C��D��
���� ���⿼���˻�ѧƽ��ļ���������Ȼ�ѧ����ʽ��д��ԭ���ԭ���͵缫��Ӧ�ķ����жϣ����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

| A�� | һ�������´ﵽ��ѧƽ��ʱ��N2��H2��NH3�����ʵ���Ũ��֮��Ϊ1��3��2 | |
| B�� | �ﵽ��ѧƽ��ʱ��N2��ȫת��ΪNH3 | |
| C�� | �ﵽ��ѧƽ��ʱ������Ӧ���淴Ӧ������ȣ��Ҷ�Ϊ�� | |
| D�� | ��λʱ��������amolN2ͬʱ����2amolNH3��˵���÷�Ӧ�Ѵﵽƽ��״̬ |
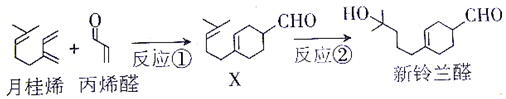
| A�� | ��Ӧ�����ڼӳɷ�Ӧ | |
| B�� | ������ȩ�ܷ�����ȥ��Ӧ | |
| C�� | ����������ȩ���Ƿ����ȩX��������ˮ | |
| D�� | 1mol������ȩ��ȫȼ��������17.5mol O2 |
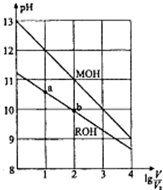 Ũ�Ⱦ�Ϊ0.10mol/L�������ΪV0��MOH��ROH��Һ���ֱ��ˮϡ�������V��
Ũ�Ⱦ�Ϊ0.10mol/L�������ΪV0��MOH��ROH��Һ���ֱ��ˮϡ�������V��pH��lg$\frac{V}{{V}_{0}}$�ı仯��ͼ��ʾ������������ȷ���ǣ�������
| A�� | MOH�ļ�������ROH�ļ��� | |
| B�� | ROH�ĵ���ƽ�ⳣ����b�����a�� | |
| C�� | ������Һ����ϡ�ͣ������ǵ�c��OH-������� | |
| D�� | ��lg$\frac{V}{{V}_{0}}$=2ʱ��������Һͬʱ�����¶ȣ���$\frac{c��{M}^{+}��}{c��{R}^{+}��}$��С |
��������
| A�� | 2H2��g��+O2��g���T2H2O ��l����H=-285.8 kJ•mol-1 | |
| B�� | 2H2��g��+O2��g���T2H2O ��l����H=+571.6 kJ•mol-1 | |
| C�� | 2H2��g��+O2��g���T2H2O ��g����H=-571.6 kJ•mol-1 | |
| D�� | H2��g��+1/2O2��g���TH2O ��l����H=-285.8 kJ•mol-1 |
| A�� | ��������ˮ����ҪĿ�����������ʳ��� | |
| B�� | ���ϡ����ά���ϳ��������л��߷��ӻ����� | |
| C�� | ����ߵ�ú�м���ʯ�ң��ɼ���ȼú�Դ�������Ⱦ | |
| D�� | ���ö�����̼��ԭ�Ϻϳɵľ۶�����̼�ɽ������������ڼ��ٰ�ɫ��Ⱦ |