题目内容
20.元素在周期表中位置,反映了元素的原子结构和元素的性质,如图是元素周期表的一部分,请用元素符号或化学式填空(1)如图所示元素中:
①金属性最强的元素是Pb;②原子半径最小的是F;③最高价含氧酸酸性最强的是HClO4;④易升华的单质是I2;⑤阴影部分属于元素周期表中的VIA族.

(2)根据元素周期律推导:
①稳定性:H2S<HCl;(用“>”或“<”表示,下同);
②沸点:HF>HCl;
③还原性:I->Br-.
(3)根据同主族元素性质的相似性和递变性进行预测:
①关于Se的预测正确的是BC(填序号)
A.常温下Se单质是气体 B.Se最高价氧化物的水化物能和NaOH发生反应
C.氢化物的化学式为H2Se D.常见的氧化物只有SeO3
②已知Cl2在水溶液中能和SO2反应,Cl2+2H2O+SO2═H2SO4+2HCl,写出Br2在水溶液中和SO2反应的离子反应方程式Br2+2H2O+SO2═4H++SO42-+Cl-.
分析 (1)①同一周期中,元素的金属性随着原子序数的增大而减弱,同一主族中,元素的金属性随着原子序数的增大而增强;
②同一周期中,原子半径随着原子序数的增大而逐渐减小,同一主族中,原子半径随着原子序数的增大而增大;
③元素的非金属性越强,其最高价氧化物的水化物酸性越强;
④易升华的单质是碘单质;
⑤主族元素中,原子最外层电子数与其族序数、最高化合价数相等;
(2)①元素的金属性越强,其氢化物越稳定;
②同一主族中,含有氢键的物质沸点大于其相邻元素氢化物的沸点;
③同一主族中,阴离子的还原性随着原子序数的增大而增强;
(3)①根据同一主族元素性质的相似性和递变性解答;
②溴和二氧化硫反应氧化还原反应生成硫酸和氢溴酸.
解答 解:(1)①根据元素周期律知,金属性最强的元素在元素周期表左下角,为Pb元素,故答案为:Pb;
②根据元素周期律知,原子半径最小的元素在元素周期表右上角,为F元素,故答案为:F;
③元素的非金属性越强,其最高价氧化物的水化物酸性越强,F和O元素没有最高价含氧酸,所以最高价含氧酸酸性最强的元素是Cl,对应的酸是HClO4,
故答案为:HClO4;
④易升华的单质是碘单质,故答案为:I2;
⑤主族元素中,原子最外层电子数与其族序数、最高化合价数相等,这几种元素原子的最外层电子数是6,所以这几种元素位于第VIA族,故答案为:VIA;
(2)①元素的金属性越强,其氢化物越稳定,S的非金属性小于Cl元素,所以稳定性:H2S<HCl,故答案为:<;
②同一主族中,含有氢键的物质沸点大于其相邻元素氢化物的沸点,HF中含有氢键,HCl中没有氢键,所以沸点HF>HCl,故答案为:>;
③同一主族中,阴离子的还原性随着原子序数的增大而增强,所以还原性I->Br-,故答案为:>;
(3)①A.硫为固体,所以常温下Se单质是固体,故A错误;
B.Se最高价氧化物的水化物为酸,能和NaOH发生中和反应,故B正确;
C.同一主族中,其氢化物的化学式相同,所以Se氢化物的化学式为H2Se,故C正确;
D.根据硫的氧化物知,常见的氧化物有SeO3和SeO2,故D错误;
故选BC;
②溴具有强氧化性,能将二氧化硫氧化为硫酸,同时自身被还原为氢溴酸,离子反应方程式为Br2+2H2O+SO2═4H++SO42-+Cl-,
故答案为:Br2+2H2O+SO2═4H++SO42-+Cl-.
点评 本题考查了元素周期表和元素周期律的综合应用,根据元素周期律来分析解答,熟练掌握元素周期律是解本题关键,比较容易.

| A. | 苯 | B. | 苯酚 | C. | 乙酸 | D. | 甲醛 |
①Cl2-消毒剂 ②Si-半导体材料 ③AgI-人工降雨
④SiO2-光导纤维 ⑤碘-预防甲状腺肿大 ⑥淀粉-检验I2的存在
⑦Ca(ClO)2-漂白纺织物 ⑧MgO-耐火材料.
| A. | ②③④⑤⑥⑧ | B. | ①②③④⑤⑧ | C. | ②③④⑤⑧ | D. | 全部 |
| A. | 铝粉投入到少量NaOH溶液中:2Al+2OH-═2AlO2-+H2↑ | |
| B. | 氯气和水反应:Cl2+H2O═2H++Cl-+ClO- | |
| C. | AlCl3溶液中加入足量的氨水:Al3++3OH-═Al(OH)3↓ | |
| D. | 铁粉加入三氯化铁溶液中:2Fe+Fe3+═3Fe2+ |
| A. | H2SO4 | B. | 氧化钠 | C. | 氯化钠 | D. | 氢氧化钠 |
| A. | 醋酸除去水垢:2H++CaCO3═Ca2++CO2↑+H2O | |
| B. | MnO2 与浓盐酸反应制Cl2:MnO2+4HClMn2++2Cl-+Cl2↑+2H2O | |
| C. | Na与水产生H2:Na+H2O═Na++OH-+H2↑ | |
| D. | Ca(HCO3)2溶液与少量NaOH溶液反应:HCO3-+Ca2++OH-═CaCO3↓+H2O |
| A. | 元素Z可与元素X形成共价化合物XZ2 | |
| B. | 元素X与W形成的原子个数比为1:1的化合物有很多种 | |
| C. | 元素W、X的氯化物中,各原子均满足8电子的稳定结构 | |
| D. | 元素Y的单质与氢氧化钠溶液或盐酸反应均有氢气生成 |
| A. | 制取乙烯:乙醇与浓硫酸共热至140℃ | |
| B. | 除去甲烷中的乙炔:混合气体通过酸性高锰酸钾溶液,再经浓硫酸干燥 | |
| C. | 除去苯中的苯酚:加入NaOH溶液振荡,静置分层后,除去水层 | |
| D. | 检验氯乙烷中的氯元素:氯乙烷与NaOH的水溶液共热后,加入AgNO3溶液有白色沉淀生成证明含CI- |
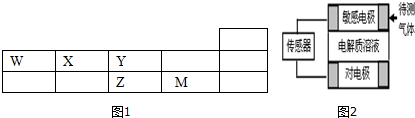
(1)M元素的离子结构示意图为

(2)Z、M二种元素的最简单气态氢化物的稳定性由强到弱的顺序为HCl>H2S(用化学式表示).
(3)NaHZ水溶液呈碱性的原因是HS-+H2?H2S+OH-((用离子方程式表示)
(4)一定条件下X2和H2在2L的密闭容器中发生如下反应X2+3H2═2XH3△H<0,测出如下数据:
| 时间(min) 物质的量(mol) | 0 | 1 | 2 | 3 | 4 |
| X2 | 3.0 | n1 | 2.4 | n3 | n5 |
| H2 | 9.0 | 8.4 | 7.2 | n4 | n6 |
| XH3 | 0 | 0.4 | n2 | 2.0 | 2.0 |
(5)在火箭推进器中装有强还原剂液态X2H4和强氧化剂液态H2Y2,当它们混合时,平均每转移1mol电子放热160kJ,同时生成液态水和氮气,该反应的热化学方程式为N2H4(l)+2H2O2(l)=N2(g)+4H2O(l)△H=-640kJ/mol.
(6)传感器工作原理如右图,气体扩散进入传感器,在敏感电极上发生反应,传感器就会接收到电信号.若待测气体WO:电解质溶液为H2ZO4,对电极充入空气,有电流产生,则下列说法中正确的是C、D
A.敏感电极作电池正极
B.对电极上的电极反应式为:O2+2H2O+4e-=4OH-
C.敏感电极附近电解质溶液的PH变小
D.若标况下44.8mLWO通入敏感电极时,传感器上电子转移数目为0.004NA.