��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣�����Ԫ���ڱ��е�λ�ã����û�ѧ����ش��������⣺
�� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
1 | �� | |||||||
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | ||||
4 | �� | |||||||
5 | �� |
��1��Ԫ����������Ϊ ��������ۺ�����Ļ�ѧʽΪ ��
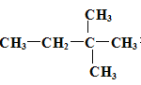
��2������������Ԫ�ص�ԭ�Ӱ�1:1��ɵij���������ĽṹʽΪ ��
��3�����������������ļ����Ӱ뾶�ɴ�С��˳��Ϊ ���������ӷ��ű�ʾ��
��4��������������������ˮ����֮�䷢����Ӧ�����ӷ���ʽ ��
��5���õ���ʽ��ʾ��������ɵĻ�������γɹ��� ��
��6�������к�����Ԫ�أ��������к��и�Ԫ�صļ������ӣ��������ữ�£�����˫��ˮ��������Ϊ���ʡ�д���÷�Ӧ�����ӷ���ʽ ��
���𰸡���1������HBrO4��2��H-O-O-H��3��Cl����O2����Mg2+��Al3+
��4��Al(OH)3��OH��AlO2����2H2O��5��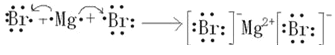
��6��2H++H2O2+2I��=2H2O+I2
�������������������1��Ԫ����λ�ڵ�2���ڵ���A�壬����ǵ�Ԫ�أ�Ԫ����λ�ڵ�4���ڵ���A�壬����Ԫ�أ������Ϊ+7�ۣ������ۺ�����Ļ�ѧʽΪHBrO4����2������HԪ�أ�����OԪ�أ�����Ԫ�ص�ԭ�Ӱ�1:1��ɳ����Ļ�����H2O2����ṹʽΪ��H-O-O-H����3�����������������ļ����ӷֱ�Ϊ��Mg2+��Al3+ ��O2����Cl�������ں�����Ӳ���Խ�࣬�뾶Խ�����Cl���뾶�����������Ų���ͬʱ���˵����Խ�뾶ԽС��������Ӱ뾶Cl����O2����Mg2+��Al3+����4������NaԪ�أ�������������Ӧˮ����ΪNaOH������AlԪ�أ�������������Ӧˮ����ΪAl(OH)3�����߷�����Ӧ�����ӷ���ʽΪ��
Al(OH)3��OH��AlO2����2H2O����5������MgԪ�أ�����BrԪ�أ������ܹ���ɻ�����MgBr2���õ���ʽ��ʾ���γɹ���Ϊ��
��6�������к��е�Ԫ�أ��������к��е����ӣ��������ữ�£�����˫��ˮ������������Ϊ�ⵥ�ʣ����ӷ���ʽΪ��2H++H2O2+2I��=2H2O+I2��