��Ŀ����
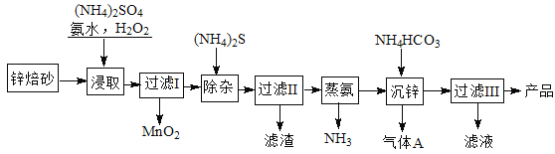
����Ŀ�����������dz��õĴ������������ͻ��Ե缫���ϡ������̲��ŷḻ���̽�˿�����Ҫ�ɷ���MnO2��1991����Allen�����о�����������ϴ��ʹ�ò�ͬ�ķ������Ʊ�������MnO2�����Ʊ���������ͼ��ʾ��

��֪����Ӧ����ʯīΪ�缫�������������Һ�ƶ������̣���Ӧ�������Ǹ���������������̣�Ҳ��������������������̡������ƶϲ���ȷ����( )
A����Ӧ�������ӷ���ʽΪMnO2��H2O2��2H��===Mn2����2H2O��O2��
B����Ӧ����������ӦʽΪMn2����2e����2H2O===MnO2����4H��
C��������KClO3����Ӧ��Ϊ2ClO��5Mn2����4H2O===5MnO2����Cl2����8H��
D��������KMnO4����Ӧ��Ϊ3Mn2����2MnO��2H2O===5MnO2����4H��
���𰸡�B
��������
���������A���������̺�˫��ˮ��Ӧ���������ӣ���Ԫ�صĻ��ϼ۽��ͣ�����������е���Ԫ�ػ��ϼ����ߣ������ж��������������ݵ����غ�͵���غ���������ӷ���ʽ��ȷ����A��ȷ��B�������õ����ӣ����ϼ۽��ͣ���B����C����������أ�ʹ+2�۵������ӱ�����Ϊ�������̣�������е���Ԫ�ػ��ϼ۽����������������ݵ���غ�͵����غ���������ӷ���ʽ��ȷ����C��ȷ��D�������������أ��������������ɶ������̣����ڹ��з�Ӧ����D��ȷ��

����Ŀ���±���Ԫ�����ڱ���һ���֣�����Ԫ���ڱ��е�λ�ã����û�ѧ����ش��������⣺
�� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
1 | �� | |||||||
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | ||||
4 | �� | |||||||
5 | �� |
��1��Ԫ����������Ϊ ��������ۺ�����Ļ�ѧʽΪ ��
��2������������Ԫ�ص�ԭ�Ӱ�1:1��ɵij���������ĽṹʽΪ ��
��3�����������������ļ����Ӱ뾶�ɴ�С��˳��Ϊ ���������ӷ��ű�ʾ��
��4��������������������ˮ����֮�䷢����Ӧ�����ӷ���ʽ ��
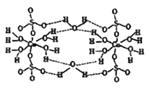
��5���õ���ʽ��ʾ��������ɵĻ�������γɹ��� ��
��6�������к�����Ԫ�أ��������к��и�Ԫ�صļ������ӣ��������ữ�£�����˫��ˮ��������Ϊ���ʡ�д���÷�Ӧ�����ӷ���ʽ ��