��Ŀ����
����Ŀ��һ���¶��£������������Ϊ1.0 L�ĺ����ܱ������з�����Ӧ��2CH3OH(g)![]() CH3OCH3(g)+H2O(g)
CH3OCH3(g)+H2O(g)
������� | �¶�(��) | ��ʼ���ʵ���(mol) | ƽ�����ʵ���(mol) | |
CH3OH(g) | CH3OCH3(g) | H2O(g) | ||
�� | 387 | 0.20 | 0.080 | 0.080 |
�� | 387 | 0.40 | ||
�� | 207 | 0.20 | 0.090 | 0.090 |
A.�÷�Ӧ������ӦΪ���ȷ�Ӧ
B.��ƽ��ʱ����������CH3OCH3��Ũ�ȴ���0.16 mol/L
C.��ƽ��ʱ����������![]() ���������еĴ�
���������еĴ�
D.����ʼʱ���������г���CH3OH(g)0.30 mol��CH3OCH3(g)1.50 mol��H2O(g)0.30 mol����Ӧ�����淴Ӧ�������
���𰸡�AD
��������
A���Ա�I�����֪�������¶�CH3OCH3(g)�����ʵ�����С��˵�������¶ȣ�ƽ�������ƶ����������¶ȣ���ѧƽ�������ȷ�Ӧ�ƶ����ʸ÷�Ӧ������ӦΪ���ȷ�Ӧ��A��ȷ��
B�����ЧΪ����ƽ�������ѹǿ����һ�����÷�Ӧ�������������ķ�Ӧ������ѹǿ��ѧƽ�ⲻ�ƶ�������������е�CH3OH����������������е���ȡ�I��ƽ��ʱCH3OCH3�����ʵ�����0.080 mol������������CH3OCH3�����ʵ���Ϊn(CH3OCH3)=2��0.080 mol=0.16 mol����Ӧ������1 L����II�ﵽƽ��ʱCH3OCH3�����ʵ���Ũ��Ϊc(CH3OCH3)=![]() =0.16 mol/L��B����
=0.16 mol/L��B����
C��II�дﵽƽ��ʱ��c(CH3OCH3)=![]() =0.16 mol/L��c(CH3OH)=
=0.16 mol/L��c(CH3OH)=![]() =0.08 mol/L��
=0.08 mol/L��![]() =0.5��III�дﵽƽ��ʱ��c(CH3OCH3)=
=0.5��III�дﵽƽ��ʱ��c(CH3OCH3)=![]() =0.090 mol/L��c(CH3OH)=
=0.090 mol/L��c(CH3OH)=![]() =0.020 mol/L��
=0.020 mol/L��![]() =0.222��0.25�����Դ�ƽ��ʱ����������
=0.222��0.25�����Դ�ƽ��ʱ����������![]() ���������е�С��C����
���������е�С��C����
D����������ƽ��ʱc(CH3OCH3)=c(H2O)=![]() =0.080 mol/L��c(CH3OH)=
=0.080 mol/L��c(CH3OH)=![]() =0.04 mol/L���������л�ѧƽ�ⳣ��K1=
=0.04 mol/L���������л�ѧƽ�ⳣ��K1=![]() =4������ʼʱ���������г���CH3OH(g)0.30 mol��CH3OCH3(g)1.50 mol��H2O(g)0.30 mol�������������ݻ���1 L�������ʵ�Ũ���������ʵ�������ֵ����ȣ���ʱŨ����Qc=
=4������ʼʱ���������г���CH3OH(g)0.30 mol��CH3OCH3(g)1.50 mol��H2O(g)0.30 mol�������������ݻ���1 L�������ʵ�Ũ���������ʵ�������ֵ����ȣ���ʱŨ����Qc=![]() =5��4=K����Ӧ�����淴Ӧ������У�D��ȷ��
=5��4=K����Ӧ�����淴Ӧ������У�D��ȷ��
�ʺ���ѡ����AD��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�����Ŀ��ijͬѧ�о�����п���Ȼ�ͭ��Һ֮�䷴Ӧ����ʵ���и�ͬѧ�۲쵽�������У�
i.�����������ݣ�ii.�к�ɫ�������ɣ�iii.��Һ�в�����ɫ������
Ϊ�˽�����������ijͬѧ�������ϣ����������Ϣ��
��� | ��ѧ��Ӧ���ӷ���ʽ |
1 | Zn+Cu2+ |
2 | Zn+2Cu2+ |
3 | Cu++2Cl- |
4 | Cu++Cl- |
��1���������ӷ���ʽ���Ͳ����������ݵ�ԭ��___��
��2��Zn��CuCl2��Ӧ���ɰ�ɫ���������ӷ���ʽ��___��
��3��Ϊ��̽��Ӱ�����ɰ�ɫ���������أ���ͬѧ��һ��ʵ�顣ȡ��ͬŨ��CuCl2��Һ������п���������̲����������ݺͺ�ɫ���壬����ʵ���������¡�
| ��� | Ũ��(rnol/L) | �Լ���п�������� | ʵ������ |
a | 0.5 | пƬ | ���̳���������ɫ���� | |
b | 1 | пƬ | ���̳��ְ�ɫ���� | |
1 | п�� | ���̳��ִ�����ɫ���� | ||
d | 1 | пƬ������NaCl���� | ������ɫ��������Ѹ���ܽ� |
�ٶԱ�ʵ��a��b��ʵ�������___��
��ijͬѧ�ӻ�ѧƽ��ĽǶȷ�����d�а�ɫ�����ܽ���ܵ�ԭ����___�������ӷ���ʽ��ʾ����Ϊ֤����ͬѧ������ԭ����ȷ����b�Թ��м�������___�����۲쵽___��֤����ͬѧ������ԭ����ȷ��
��4�����ó�������ȥ������ʵ��������Ӧ�ù㷺��
���ڹ�ҵ��ұ��п��Ϊ�˳�ȥZnSO4���Һ��Һ�е�C1-���ɼ���___��___�����ɳ�������ȥ��
��ͨ����һ���������ϵ�֪��CuCl���γ�����Һ��pH��Cu2+������Ũ���йأ�һ�������£�ͨ��ʵ��ó�pH��Cu2+Ũ�ȶ�Cl-������Ӱ����ͼ��ʾ��
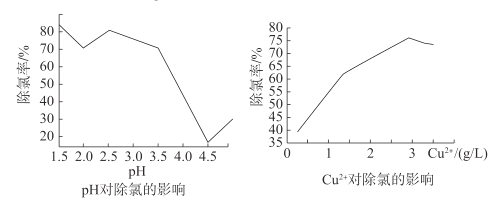
����ͼ����ȥ�õ��Һ��Cl-�����������___��