��Ŀ����
3����Ȼ���У�����������������������������������ã�������ͭ��Cu2S����ͭ����CuS������֪��Cu2S��CuS�����ֲ�����ˮ�ĺ�ɫ���壬��һ�������¶�����ϡHNO3��Ӧ��
��3CuS+8H++8NO3-��3Cu2++3SO42-+8NO��+4H2O
��3Cu2S+16H++10NO3-��6Cu2++3SO42-+10NO��+8H2O
�ֽ��ķ�������ͬ��ijCu2S��CuS�������Ʒ�ֱ��100mL���ʵ���Ϊ5mol/L��ϡ�����ַ�Ӧ����ȡ��Ʒ����������������������״���ⶨ�������ʾ��
| ʵ���� | a | b | c |
| ��Ʒ������g�� | 9.6 | 12.8 | 64.0 |
| ���������L�� | 5.04 | 6.72 | V |
��1��aʵ���������Һ��c��NO3-��=2.75mol/L��
��2��bʵ���������Һ��pH=0��
���� ��1�����ݷ�Ӧ��֪��������������ʵ�����������������ӵ����ʵ�����ȣ��ݴ˼������Ӧ��a��Һ�����������Ũ�ȣ�
��2������a�������ж�b�л����ǡ����ȫ��Ӧ��Ȼ������������Cu2S��CuS�����ʵ������ֱ������������������������ʵ�����ʽ�����Cu2S��CuS�����ʵ������ٸ��ݷ�Ӧ����ʽ�������Ӧ�����������ӵ����ʵ�����Ȼ�����c=$\frac{n}{V}$���������������Ũ�ȣ��������ʣ��������Ũ�ȼ���Һ��pH��
��� �⣺��1��100mL���ʵ���Ϊ5mol/L��ϡ�������ĺ�����������ʵ���Ϊ��5mol/L��0.1L=0.5mol��
���ݷ�Ӧ��3CuS+8H++8NO3-��3Cu2++3SO42-+8NO��+4H2O����3Cu2S+16H++10NO3-��6Cu2++3SO42-+10NO��+8H2O��֪����Ӧ����NO��������ʵ�����������������ӵ����ʵ�����ȣ�aʵ��������NO�����ʵ���Ϊ��$\frac{5.04L}{22.4L/mol}$=0.225mol�����������Ũ��Ϊ��$\frac{0.225mol}{0.1L}$=2.25mol/L����Ӧ����ҺŨ��Ϊ5mol/L-2.25mol/L=2.75mol/L��
�ʴ�Ϊ��2.75mol/L��
��2��9.6g���������5.04LNO���壬12.8g������ܹ�����һ�����������Ϊ��$\frac{12.8g}{9.6g}$��5.04L=6.72L��˵��b�л������ȫ��Ӧ��
��12.8g����Ʒ��CuS�����ʵ���Ϊx��Cu2S�����ʵ���Ϊy��
���������ɵã���96x+160y=12.8��
������������ɵã���$\frac{8}{3}$x+$\frac{10}{2}$y=$\frac{6.72L}{22.4L/mol}$=0.3��
���ݢ٢ڽ�ã�x=y=0.05mol��
���ݷ���ʽ��3CuS+8H++8NO3-��3Cu2++3SO42-+8NO��+4H2O����3Cu2S+16H++10NO3-��6Cu2++3SO42-+10NO��+8H2O��֪���������ӵ����ʵ���Ϊ��n��H+��=0.05mol��$\frac{8}{3}$+0.05mol��$\frac{16}{3}$=0.4mol��
�����������ӵ�Ũ��Ϊ��c��H+��=$\frac{0.4mol}{0.1L}$=4mol/L��
����ʣ��������Ũ��Ϊ��c��H+��=5mol/L-4mol/L=1mol/L����Ӧ��b��Һ��pH=0��
�ʴ�Ϊ��0��
���� ���⿼���˻���ﷴӦ�ļ��㣬��Ŀ���ܽϴ���ȷ�ж�b�л�����Ƿ���ȫ��ӦΪ���ؼ���ע�����������غ㶨���ڻ�ѧ�����е�Ӧ�ã�����������ϴ�����������ѧ���ķ�����������ѧ����������

| A�� | 3�� | B�� | 4�� | C�� | 5�� | D�� | 6�� |
| A�� | Ag+ | B�� | SO42- | C�� | CH3COO- | D�� | Mg2+ |
| A�� | CH2O��C2H4O2 | B�� | C4H10��C4H6O | C�� | CF2Cl2��C2F2Cl2 | D�� | C4H10��C3H6 |
| A�� | ��Һ©��ʱӦ�ر��䲣�����ͻ��� | |
| B�� | ��ʪ��ĵ⻯�ص�����ֽ����Br2��g����NO2 | |
| C�� | ��50mL��Ͳ������0.1000mol•L-1̼������Һ | |
| D�� | ����NH4+ʱ���������м���NaOH��Һ���ȣ���ʪ�����ɫʯ����ֽ�����ݳ������� |
 ���������˵���в���ȷ���ǣ�������
���������˵���в���ȷ���ǣ�������| A�� | һ���������ܷ���������Ӧ | |
| B�� | һ���������ܷ�����������Ӧ | |
| C�� | ���ܷ����ӳɷ�Ӧ | |
| D�� | 1mol��������Na2CO3��Һ��Ӧ�������1.5mol Na2CO3 |
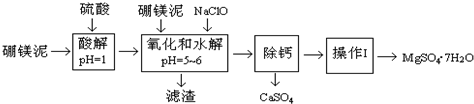
��֪��NaClO��Mn2+��Ӧ����MnO2������
| ������ | Fe��OH��3 | Al��OH��3 | Fe��OH��2 |
| ��ʼ����pH | 2.3 | 4.0 | 7.6 |
| ��ȫ����pH | 4.1 | 5.2 | 9.6 |
��1��ʵ��������1.00mol/L������80.0mL������98%��Ũ���������ƣ�����Ͳ������������ͷ�ι��⣬����Ҫ�IJ����������ձ���100mL����ƿ��
��2����������Ҫ�ɷݳ�����Fe��OH��3��Al��OH��3�⣬����MnO2��SiO2��
��3�������NaClO����Mn2+��Ӧ����MnO2�������÷�Ӧ�����ӷ���ʽ��Mn2++ClO-+H2O=MnO2��+2H++Cl-��
�ڵ���pH=5-6֮ǰ������һ������Ҳ�ᱻNaClO�������÷�Ӧ�����ӷ���ʽΪ��2Fe2++ClO-+2H+=2Fe3++Cl-+H2O��
��4��Ϊ�˼�����Һ��Fe3+�Ƿ�������ѡ�õ��Լ���A��
A��KSCN��Һ B������KI��Һ C��H2O2 D��KMnO4ϡ��Һ
��5����֪MgSO4��CaSO4���ܽ�����±���
| �¶ȣ��棩 | 40 | 50 | 60 | 70 |
| MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
| CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
��6���������ṩ����þ�������Ϊ100.0g���õ���MgSO4•7H2O196.8g����MgSO4•7H2O�IJ���Ϊ80.0%����Է���������MgSO4•7H2O-246 MgO-40����

��֪����1��������Ӧ�ĵ�һ�����е�Ѹ�ٶ���ȫ���ڶ�����Ӧ���棬���ѽ��У�
��2���й����ʵ��������±���ʾ��
| ���� | ���ʣ��е㼰�ֽ��¶Ⱦ�Ϊ101kPa��ã� |
| �ڱ��������� ��M=148g/mol�� | ��ɫ��״���壮��������ˮ��������ˮ���л��ܼ��� �ܶ�1.53g/cm3���е�295�森 |
| ��������M=74g/mol�� | ��ɫҺ�壮����ˮ�������л��ܼ����ܶ�0.81g/cm3���е�117.7�森����ˮ�γɶ�Ԫ������е�92.7�棩�� |
| �ڱ������������ ��M=278g/mol�� | ��ɫ��״Һ�壮������ˮ�������л��ܼ����ܶ�1.49g/cm3���е�340�森����������180�����������ֽ⣮ |
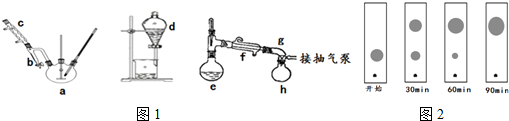
�����Ʊ��ֲ�Ʒ����������ƿ��ڲ����¶ȼƣ���һ������ӷ�ˮ���������ܣ����м���ȼ���3g��0.02mol���ڱ�����������������ʯ������ҡ�»�������6.5mL��0.07mol����������0.1mLŨ����Ļ��Һ����װ����װ�ã��ڷ�ˮ���м�����������֧��ƽ�룮�������£�ʹ������У���ƿ�ڹ�����ȫ��ʧ��������������ˮ������СҺ�γ���ײ������¶�����140��ʱ���ֹͣ���ȣ�
���ֲ�Ʒ����������ӦҺ��ȴ��70������ʱ�������Һת���Һ©��������10mL 5% Na2CO3��Һϴ�ӣ��л�����15mL���ȵı���ʳ��ˮϴ��2��3�Σ����л�������ԣ����������״�����ˮ����þ�����ȥ��������л�������ȥ������������������ڳ����õļ�ѹ�������ռ�180��190�����ּ��ò�Ʒ�������������ش��������⣺
��1������e������������ƿ��Ũ����������Ǵ�������ˮ�������õ������ǿɽ����л���ķе㣬���Է�ֹ�л�����ˮ̼������߲���Ĵ��ȣ�
��2���Ʊ�װ��������ˮ����������������������������ͬ���Ʊ������з�Ӧ���е��յ�ı�־�Ƿ�ˮ���е�ˮλ���ٷ����仯��
��3����Ʒ���������У�����Na2CO3��Һ��Ŀ���ǽ������
 ת����Σ��Ӷ��������룬�ò����Ƿ�ɸ���NaOH��Һ������ǡ�����ԭ���ǣ��������Ƽ���̫ǿ����ʹ�ڱ����������������ˮ�⣻������ʳ��ˮϴ��һ�����Ƿ�ֹ�л�����黯�������ڷֲ㣬��һ������Ϊ�˽����ڱ���������������ܽ�ȣ�
ת����Σ��Ӷ��������룬�ò����Ƿ�ɸ���NaOH��Һ������ǡ�����ԭ���ǣ��������Ƽ���̫ǿ����ʹ�ڱ����������������ˮ�⣻������ʳ��ˮϴ��һ�����Ƿ�ֹ�л�����黯�������ڷֲ㣬��һ������Ϊ�˽����ڱ���������������ܽ�ȣ���4��ʵ���л��ɲ��ñ���ɫ����ԭ���Ͳ�����ֽ������ͬ�����ٷ�Ӧ���̣��ֱ��ڷ�Ӧ��ʼ��IJ�ͬʱ�䣬��ëϸ�ܴ�������ƿ��ȡ��������������ɫ��չ����������������£��������ṹ�����ʿ���ɫ���ߵ���ͼ2������Ϊ�����ܳ��ֵ������D��
A����ʼ B��30min C��60min D��90min��
| A�� | ij����Ԫ�شӻ���̬��Ϊ����̬ʱ����Ԫ��һ��������ԭ��Ӧ | |
| B�� | ������Ԫ�ص����ӣ�һ������������ | |
| C�� | ���������ӱ���ԭһ���õ��������� | |
| D�� | ��������ԭ��Ӧ�У���������һ������ԭ |