��Ŀ����
16��A��һ������ҩ����м��壬��ṹ��ʽ��ͼ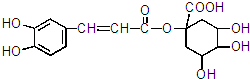 ��ʾ��
��ʾ��A��ˮ������E��F��F���б�������ϳ�·�����£�
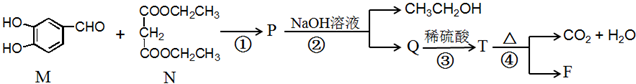
����P��Q��T�ֱ����һ���л���ϳ�·���в��ֲ��P��Ӧ��������ȥ����֪��

��ش��������⣺
��1������A�Ľṹ�ƶϣ�A��ab������ĸ��ţ���
a��ʹ������Ȼ�̼��Һ��ɫ b����̼��������Һ��Ӧ�ų����� c����������Һ����������Ӧ
��2��M�еĹ������������ǻ���ȩ����N�ĺ˴Ź���������3�ַ壮
��3��E������ȥ��Ӧ�������ɿ�������ҩ���ԭ��G��C7H10O5����G�Ľṹ�У�̼̼˫������������̼ԭ�ӣ�G�Ľṹ��ʽ��
 ��
����4���ڢڲ���Ӧ�Ļ�ѧ����ʽ��
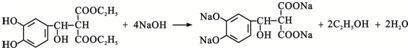 ��
����5���ڢܲ���Ӧ�Ļ�ѧ����ʽ��
 ��
��
���� A�к��б��������ǻ���̼̼˫�����������Ȼ������ǻ������б����ӡ�ϩ�����������ᡢ�������ʣ�Aˮ������E��F��F�к��б�������EΪ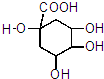 ��FΪ
��FΪ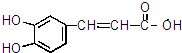 ��M��N������Ӧ����P�����������Ϣ֪��PΪ
��M��N������Ӧ����P�����������Ϣ֪��PΪ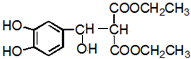 ��Pˮ�������Ҵ���Q��Q�ṹ��ʽΪ
��Pˮ�������Ҵ���Q��Q�ṹ��ʽΪ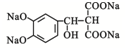 ��Q�ữ�õ�T��T�ṹ��ʽΪ
��Q�ữ�õ�T��T�ṹ��ʽΪ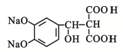 ��T���ȷֽ����ɶ�����̼��ˮ��F���ݴ˷������
��T���ȷֽ����ɶ�����̼��ˮ��F���ݴ˷������
��� �⣺A�к��б��������ǻ���̼̼˫�����������Ȼ������ǻ������б����ӡ�ϩ�����������ᡢ�������ʣ�Aˮ������E��F��F�к��б�������EΪ ��FΪ
��FΪ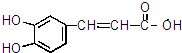 ��M��N������Ӧ����P�����������Ϣ֪��PΪ
��M��N������Ӧ����P�����������Ϣ֪��PΪ ��Pˮ�������Ҵ���Q��Q�ṹ��ʽΪ
��Pˮ�������Ҵ���Q��Q�ṹ��ʽΪ ��Q�ữ�õ�T��T�ṹ��ʽΪ
��Q�ữ�õ�T��T�ṹ��ʽΪ ��T���ȷֽ����ɶ�����̼��ˮ��F��
��T���ȷֽ����ɶ�����̼��ˮ��F��
��1��A�к��б��������ǻ���̼̼˫�����������Ȼ������ǻ������б����ӡ�ϩ�����������ᡢ�������ʣ�
a������̼̼˫���������ܺ��巢���ӳɷ�Ӧ��ʹ������Ȼ�̼��Һ��ɫ������ȷ��
b�������Ȼ�����������̼��������Һ��Ӧ�ų����壬����ȷ��
c������ȩ�������Բ���������Һ����������Ӧ���ʴ���
��ѡab��
��2����M�к��й������������ǻ���ȩ����N�к���������ԭ�ӣ����Ժ˴Ź������������֣��ʴ�Ϊ���ǻ���ȩ����3��
��3��EΪ ��E������ȥ��Ӧ�������ɿ�������ҩ���ԭ��G��C7H10O5����G�Ľṹ�У�̼̼˫������������̼ԭ�ӣ�G�Ľṹ��ʽ��
��E������ȥ��Ӧ�������ɿ�������ҩ���ԭ��G��C7H10O5����G�Ľṹ�У�̼̼˫������������̼ԭ�ӣ�G�Ľṹ��ʽ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��PΪ ��Pˮ�������Ҵ���Q��Q�ṹ��ʽΪ
��Pˮ�������Ҵ���Q��Q�ṹ��ʽΪ ��P����Q��Ӧ����ʽΪ
��P����Q��Ӧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��5��T�ṹ��ʽΪ ��T���ȷֽ����ɶ�����̼��ˮ��F����Ӧ����ʽΪ
��T���ȷֽ����ɶ�����̼��ˮ��F����Ӧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϣ�Ϊ�߿���Ƶ�㣬��֪�����ż������ʹ�ϵ�ǽⱾ��ؼ���ͬʱ������ѧ��֪ʶǨ����������������Ϣ����Ӧ���������ƶϣ���Ŀ�Ѷ��еȣ�

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д� ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д�| A�� | �������ʵ�����NaHC2O4��Na2C2O4����Һ��2c��Na+ ��=3[c��HC2O4- ��+c��C2O42-��+c��H2C2O4��] | |
| B�� | pH=4.3��NaHC2O4��Һ�У�c��Na+ ����c��HC2O4-����c��H2C2O4����c��C2O42-�� | |
| C�� | 0.10mol•L-1NaHCO3��Һ�У�c��Na+ ��+c��H+ ��=c��OH- ��+c��HCO3-��+c��CO32-�� | |
| D�� | 0.10mol•L-1 NaHCO3��Һ��ˮϡ�ͺ�n��H+ ����n��OH- ���ij˻����� |
| A�� | �ڶ���ӵ�ԭ��������ߵĵ���ͨ������˽��������ڻ | |
| B�� | ����������������������͵ĵ��Ӳ��ϣ�����ֻ��������M������N�� | |
| C�� | ����������������������͵ĵ��Ӳ��� | |
| D�� | ���������ֻ����8�����Ӳ��ȶ� |
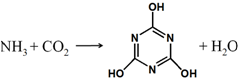 CO2����Դ�������ǽ������ЧӦ����Ҫ;������ͼ����һ����������NH3����CO2������Ҫ������Ʒ��������ķ�Ӧ�������й����������˵����ȷ���ǣ�������
CO2����Դ�������ǽ������ЧӦ����Ҫ;������ͼ����һ����������NH3����CO2������Ҫ������Ʒ��������ķ�Ӧ�������й����������˵����ȷ���ǣ�������| A�� | ����ʽΪC3H6N3O3 | B�� | �����мȺ��ЦҼ��ֺ��Цм� | ||
| C�� | �����мȺ����Լ����ֺ��Ǽ��Լ� | D�� | ���ɸ����ʵ�������ӦΪ�кͷ�Ӧ |
| A�� | ��Fe��NO3��2��KI�����Һ�м�������ϡ���3Fe2++4H++NO3-=3Fe3++2H2O+NO�� | |
| B�� | ��ĭ����������ԭ����2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2�� | |
| C�� | ����������Һ�м��������İ�ˮ��Ag++2NH3•H2O=Ag��NH3��2++2H2O | |
| D�� | �������İ�ˮ�������Ṥҵ��β����SO2+NH3•H2O=NH4++HSO3- |
| A�� | ԭ�Ӱ뾶��A��D��C��B | |
| B�� | ����⻯����ȶ��ԣ�D��C | |
| C�� | A��C�γɵĻ���������ˮ������Һ�Լ��� | |
| D�� | B��D�γɵĻ���������ˮ������Һ�Լ��� |
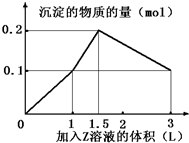 ij�����Һ�У�������X��Y��0.1mol�������еμ�0.1mol/L��Z��Һ�����ó��������ʵ�����ͼ�������������X��Y��Z�ֱ��ǣ�������
ij�����Һ�У�������X��Y��0.1mol�������еμ�0.1mol/L��Z��Һ�����ó��������ʵ�����ͼ�������������X��Y��Z�ֱ��ǣ�������| A�� | �Ȼ������Ȼ������������� | B�� | �Ȼ������Ȼ�þ���������� | ||
| C�� | ƫ�����ơ��Ȼ��������� | D�� | ƫ�����ơ��������������� |
| ѡ�� | ʵ �� Ŀ �� | ʵ �� ���� |
| A | ����Һ�н�MnO4+��ȫת��ΪMn2+ | ������KMnO4��Һ�еμ�H2O2��Һ����ɫ��ʧ |
| B | ��֤Br2��������ǿ��I2 | ��������ˮ����KI��Һ�У��ټ���CCl4�������ã��ɹ۲쵽�²�Һ�����ɫ |
| C | ����Fe��NO3��2�����Ƿ����������� | ��Fe��NO3��2��Ʒ����ϡH2SO4�μ�KSCN��Һ���۲���Һ�Ƿ��� |
| D | ��֤Fe��OH��3���ܽ��С��Mg��OH��2 | ��FeCl3��Һ����Mg��OH��2����Һ�У����ɹ۲쵽�����ɰ�ɫ��Ϊ���ɫ |
| A�� | A | B�� | B | C�� | C | D�� | D |