��Ŀ����
����Ŀ���Ƶij������ϼ�Ϊ��3�ۣ��ҹ��̲��ŷḻ�ĺ��ƿ�ʯ(Y2FeBe2Si2O10)����ҵ��ͨ�������������̿ɻ�������ơ�

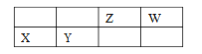
��֪���ٸ��������йؽ��������γ������������ʱ��pH���±���
���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
Fe3�� | 2.1 | 3.1 |
Y3�� | 6.0 | 8.2 |
����Ԫ�����ڱ��У���Ԫ�غ���Ԫ�ش��ڵڶ����ں͵������ڵĶԽ���λ�ã���ѧ�������ơ�
��ش��������⣺
(1)д��Na2SiO3��һ����;________________________��
(2)����Na2SiO3��Na2BeO2�����Һ���Ƶ�Be(OH)2������
�����ѡ�������________�����Լ�����ͨ����Ҫ�IJ�������ʵ�֡�
A.NaOH��Һ����������B.��ˮ����������������C.CO2����������������D.HNO3
��д��Na2BeO2���������ᷢ����Ӧ�����ӷ���ʽ_______________________________________________��
(3)ΪʹFe3��������ȫ���ð�ˮ����pH��aʱ��aӦ������_________��Χ�ڣ������Ӱ�ˮ����pH��b������Ӧ�����ӷ���ʽΪ___________________________________________��
����Fe3���Ƿ������ȫ�IJ���������_________________________________________��
(4)���ղ�����ʱ�����ֽⷴӦ����������Ϊ�����ƣ����������ʹ����ʯ��ˮ����ǡ�д��������[Y2(C2O4)3��nH2O]���յĻ�ѧ����ʽ_____________________________________��
���𰸡���ҵ�ϼ����Ʊ��轺��ľ�ķ������ B BeO22-��4H��===Be2����2H2O 3.1��6.0 Y3����3NH3��H2O===Y(OH)3����3NH4+ ȡ������Һ���μӼ���KSCN��Һ���۲���Һ�Ƿ��ΪѪ��ɫ��������Ѫ��ɫ����˵��Fe3����ȫ��������֮��δ��ȫ���� Y2(C2O4)3��nH2O![]() Y2O3��3CO����3CO2����nH2O
Y2O3��3CO����3CO2����nH2O
��������
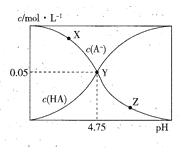
�ƿ�ʯ��Y2FeBe2Si2O10���������������ƹ��ۺ�ˮ�ܹ��˵õ�Na2SiO3��Na2BeO2��Һ������ΪY��OH��3��Fe2O3������ϡ�����ܽ����백ˮ������ҺpH��3.7<a<6.0��Χ���������ӣ�����AΪY��OH��3���������백ˮ������ҺpH��8.2���ϼ������õ�Y2��C2O4��3�����ˡ�ˮϴ�����յõ�Y2O3��ĩ������Ԫ�غ���Ԫ�ػ�ѧ�����������֣��ɷ����Ƶ�Be��OH��2�������Լ�������ʽ����������Ϣ�Ʋ���Ӧ���������д����Ӧ����ʽ��
(1)Na2SiO3ˮ��Һ�׳�ˮ������
(2)�����ڱ��У��롢����ѧ�������ƣ�����NaAlO2����ƶϣ�
��Na2BeO2���������ᷢ����Ӧ�����Ȼ��롢�Ȼ��ƺ�ˮ��
(3)Ŀ��ΪʹFe3��������ȫ������Ӱ��Y3����
(4)���ղ�����ʱ�����ֽⷴӦ����������Ϊ�����ƣ����������ʹ����ʯ��ˮ����ǣ�˵����CO2���������
(1)Na2SiO3ˮ��Һ�׳�ˮ�����������ڹ�ҵ�ϼ����Ʊ��轺��ľ�ķ�����ȣ�
(2)�����ڱ��У��롢��Ԫ�ش��ڵڶ����ں͵������ڵĶԽ���λ�ã���ѧ�������ƣ�����Na2SiO3��Na2BeO2�Ļ����Һ���Ƶ�Be(OH)2����������Na2BeO2�����ʺ�NaAlO2����ƶϣ��ӹ��������ᣬ�����Ʒ�Ӧ���ɹ��������Na2BeO2�ķ�Ӧ�����Ȼ�����Һ���ټ��������ˮ���������ӣ���ѡB��
��Na2BeO2���������ᷢ����Ӧ�����Ȼ��롢�Ȼ��ƺ�ˮ����Ӧ�����ӷ���ʽΪBeO22-��4H��===Be2����2H2O��
(3)���������ӿ�ʼ������������ȫ��pH��ΧΪ2.1��3.1�������ӿ�ʼ�����ͳ�����ȫ��pHΪ6.0��8.2������ʹFe3��������ȫ�����ð�ˮ����pH��a��3.1��a��6.0�������Ӱ�ˮ����pH��b������Ӧ�����ӷ���ʽΪY3����3NH3��H2O===Y(OH)3����3NH4+��
(4)���ղ�����ʱ�����ֽⷴӦ����������Ϊ�����ƣ����������ʹ����ʯ��ˮ����ǣ�˵����CO2���������������[Y2(C2O4)3��nH2O]���յĻ�ѧ����ʽΪY2(C2O4)3��nH2O![]() Y2O3��3CO����3CO2����nH2O��
Y2O3��3CO����3CO2����nH2O��

 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�����Ŀ��������ͼ��ʾװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50mL0.50mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50mL0.55mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У���Ͼ��ȣ���û��Һ����¶ȡ�
�ش��������⣺
(1)д���÷�Ӧ���Ȼ�ѧ����ʽ����֪����lmolҺ̬ˮ�ķ�Ӧ��Ϊ��57.3kJ/mol��______________________��
(2)�ֽ�һ������ϡ����������Һ��ϡ����������Һ��ϡ��ˮ�ֱ�� 1L1mol/L����ǡ����ȫ��Ӧ���䷴Ӧ�ȷֱ�ΪH1��H2��H3����H1��H2��H3�Ĵ�С��ϵΪ________________________��
(3)�������������������Һ���ܶȶ���1g/cm3����֪�кͷ�Ӧ��������Һ�ı�����c=4.18J/(g����)��Ϊ�˼����к��ȣ�ijѧ��ʵ���¼���������
ʵ����� | ��ʼ�¶� | ��ֹ�¶� | |
���� | ����������Һ | �����Һ | |
1 | 20.0 | 20.2 | 23.2 |
2 | 20.2 | 20.4 | 23.4 |
3 | 20.4 | 20.6 | 23.6 |
4 | 20.1 | 20.3 | 26.9 |
���ݸ�ѧ����ʵ�����ݼ��㣬��ʵ���õ��к���H_____(�������һλС��)��
(4)�����60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������__________ (���ȡ�����ȡ�)�������к���__________(���ȡ�����ȡ�)��
(5)���ü������ȼƲ�������������������������Һ�кͷ�Ӧ�ķ�Ӧ�ȣ����д�ʩ�������ʵ�龫�ȵ�����_______��
A��������Һ��(��ȷ��0.01 mL)������Ͳ(��ȷ��0.1 mL)��ȡ��ӦҺ
B�����ٽ�����Һ��ϣ����ٽ��貢��¼����¶�
C�����ڡ���Ͳ֮�����������ʣ���ֹ������ʧ
D��������Ϊ500����¶ȼƴ�������Ϊ100����¶�