��Ŀ����
��ҵ���û�ͭ��ұ��ͭ����¯���ۺ����õ�һ�ֹ����������£�
��1��ұ�������еõ�Cu2O��Cu�Ļ�����Ϊ����ͭ�����������A1�ڸ��������»�Ϸ�Ӧ�ɵô�ͭ����Ӧ��ѧ����ʽΪ________����ͭ����ʱӦ����ͭ������ֱ����Դ��____��������____���õ����Ƚϸߵľ�ͭ��
��2����ͳ��ͭ�ķ�����Ҫ�ǻ���ͭ������Ҫ��ӦΪ��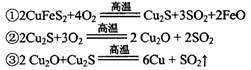
ÿ����1 mol Cu��������____mol O2����Ӧ���е���������____��
��3����ͭ������¯��(�� )���Ʊ�Fe2O3���������̻ش��������⣺
)���Ʊ�Fe2O3���������̻ش��������⣺
�ټ�������NaClO��Һ��Ŀ����_______ �������ӷ���ʽ��ʾ����
�ڳ�ȥAl3�������ӷ���ʽ��____��
��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO���ṩ���Լ��У�ϡ���ᡢϡ���ᡢKSCN��Һ��KMnO4��Һ��NaOH��Һ����ˮ����ѡ�Լ���____��ʵ����ƣ�________��
��1��3Cu2O+2Al Al2O3+6Cu (2��) �� (1��) �� (1��)
Al2O3+6Cu (2��) �� (1��) �� (1��)
��2��2.5 (1��) Cu2O��Cu2S (1��)
��3����2Fe2++ClO-+2H+=2Fe3++Cl-+H2O (2��)
��Al3++4OH-=AlO2-+2H2O (2��)
��ϡ���� ���������Һ (2��)
ȡ�������������Һ���Թ��У��μӾ�ϡ�����ܽ��¯����Һ�����������Һ����ɫ��ȥ��֤������Fe2+ (2��)
���������������1���������֪������ͭ�������A1�ڸ��������»�Ϸ�Ӧ�ɵô�ͭΪ���ȷ�Ӧ����⾫��ͭ�Ǵ�ͭ�������������ӵ�Դ��������2������Ŀ����������ʽ������ɵã�2CuFeS2+5O2=2FeO+4SO2+2Cu��ÿ����1 mol Cu��������2.5mol O2������ͭ�Ļ��ϼ۽��ͣ���Cu2O��Cu2S����������3��¯���к���FeO����Ҫ�����������������л�ԭ�ԣ��ʼ���ʱ��ѡ�����Ը�����ء�
���㣺������ҵ���̿���������ԭ֪ʶ

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���ʯ�����ȼҵ�е�һ�ַ���������������±���ʾ��
�õ�ʯ����������ˮCaCl2��ij��������������¹������̣�
��֪�Ȼ��ƾ���Ļ�ѧʽ�ǣ�CaCl2��6H2O��H2S��һ���������壬�Ҿ��л�ԭ�ԡ�
��1����Ӧ���м������Ӧѡ��___________________��
��2����ɫ����Ӧ���������X��_______________���豸A��������______________���豸B������Ϊ________________���豸C��������____________________��
��3��Ϊ�����㻷��Ҫ���轫����H2Sͨ�����ճأ��������������ʺ���Ϊ���ռ�����_____________����Ӧ�Ļ�ѧ����ʽΪ_________________��
| A��ˮ | B��Ũ���� | C��ʯ���� | D������ |
��5���ȼҵ�缫����ʽ_____________________��
����ʵ�ʲ����ڹ�ҵ�������� (����)��
| A��CO2ͨ������������Һ����Na2CO3 |
| B��H2��Cl2������HCl |
| C��Cl2ͨ�����ʯ��ˮ����Ư�� |
| D������������� |




 Na2S2O5��H2O�ȶಽ��Ӧ��
Na2S2O5��H2O�ȶಽ��Ӧ��

