��Ŀ����
����Ŀ����������������ɴ�����Ⱦ����Ҫ���ʡ��о���������ķ�Ӧ��������������������Ⱦ����Ҫ���塣�ش�����������
��1����֪2NO(g) +O2(g)![]() -2NO2(g) ��H�ķ�Ӧ���̷�������
-2NO2(g) ��H�ķ�Ӧ���̷�������
��2NO( g )![]() N2O2 (g)( �� ) ��H1<0��v1��=k1��c2(NO)��v1��=k1��c2(N2O2)
N2O2 (g)( �� ) ��H1<0��v1��=k1��c2(NO)��v1��=k1��c2(N2O2)
��N2O2 (g)+ O2 (g) ![]() 2NO2(g)(��) ��H2<0��v2��=k2��c2(N2O2)c(O2)��v2��=k2��c2(NO2)
2NO2(g)(��) ��H2<0��v2��=k2��c2(N2O2)c(O2)��v2��=k2��c2(NO2)
�ȽϷ�Ӧ���Ļ��E1�뷴Ӧ���Ļ��E2�Ĵ�С: E1__ E2 (����>������<������=��) ���ж�������__________��2NO(g) +O2(g)![]() 2NO2(g) ��ƽ�ⳣ��K��������Ӧ���ʳ���k1����k1����k2���� k2���Ĺ�ϵʽΪ_______����֪��Ӧ���ʳ���k���¶����߶������������¶Ⱥ�k2���� k2���ֱ�����a����b������a____b (����>������<������=��)��һ�������£�2NO (g)+O2(g)
2NO2(g) ��ƽ�ⳣ��K��������Ӧ���ʳ���k1����k1����k2���� k2���Ĺ�ϵʽΪ_______����֪��Ӧ���ʳ���k���¶����߶������������¶Ⱥ�k2���� k2���ֱ�����a����b������a____b (����>������<������=��)��һ�������£�2NO (g)+O2(g) ![]() 2NO2 (g)��ƽ������ߵ�ij�¶ȣ��ٴ�ƽ���v2����ԭƽ���С�������������ʷ��̷����������Ľ�����_________________��
2NO2 (g)��ƽ������ߵ�ij�¶ȣ��ٴ�ƽ���v2����ԭƽ���С�������������ʷ��̷����������Ľ�����_________________��
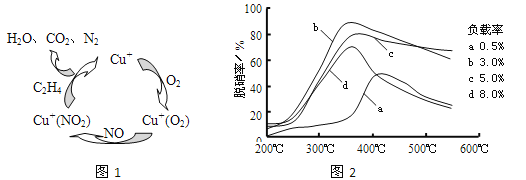
��2��������ϩ(C2H4)��Ϊ��ԭ������(NO)������������������ͼ��ʾ������Ӧ��n(NO): n(O2) =2 ��1�����ܷ�Ӧ�Ļ�ѧ����ʽΪ_______________�����������¶ȡ�������(����ɸ�д�������������) �Ĺ�ϵ������ͼ��Ϊ�ﵽ�������Ч����Ӧ���õ�������________________��
����NO��ֱ�Ӵ�NO�ֽ�����N2��O2�����䷴Ӧ������������(Vo��������Ѩ)��
2Ni2++2Vo+2NO��2Ni3++2O-+N2 2O-��O2-+1/2O2+Vo ______________
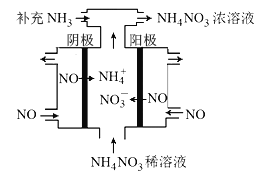
��3�����NO���Ʊ�NH4NO3���乤��ԭ����ͼ��ʾ�������ĵ缫��ӦʽΪ______________��
���𰸡� �� ���Խ��һ����ӳ�Ϊ�����Խ�ѣ���Ӧ����Խ�� ![]() �� �¶����ߣ���Ӧ�����ڵ�ƽ������������ڷ�Ӧ�ٵ����ʴ���c(N2O2)��С����̶ȴ���k2����c(O2)����ij̶���ʹ���ߵij˻���v2����С 6NO+3O2+2C2H4
�� �¶����ߣ���Ӧ�����ڵ�ƽ������������ڷ�Ӧ�ٵ����ʴ���c(N2O2)��С����̶ȴ���k2����c(O2)����ij̶���ʹ���ߵij˻���v2����С 6NO+3O2+2C2H4![]() 3N2+4CO2+4H2O 350����������3.0% 2Ni3+ + O2-��2Ni2+ +VO+
3N2+4CO2+4H2O 350����������3.0% 2Ni3+ + O2-��2Ni2+ +VO+![]() O2 NO + 6H+ +5e- = NH4+ + H2O
O2 NO + 6H+ +5e- = NH4+ + H2O
����������1����2NO(g)![]() N2O2(g)(��)����N2O2(g)+ O2(g)
N2O2(g)(��)����N2O2(g)+ O2(g) ![]() 2NO2(g)(��) �����Խ��һ����ӳ�Ϊ�����Խ�ѣ���Ӧ����Խ������Ӧ�ٵĻ��E1����Ӧ�ڵĻ��E2����2NO(g)
2NO2(g)(��) �����Խ��һ����ӳ�Ϊ�����Խ�ѣ���Ӧ����Խ������Ӧ�ٵĻ��E1����Ӧ�ڵĻ��E2����2NO(g)![]() N2O2(g)(��) ��H1<0��v1��=k1��c2(NO)��v1��=k1��c2(N2O2)����N2O2(g)+ O2(g)
N2O2(g)(��) ��H1<0��v1��=k1��c2(NO)��v1��=k1��c2(N2O2)����N2O2(g)+ O2(g) ![]() 2NO2(g)(��) ��H2<0��v2��=k2��c2(N2O2)c(O2)��v2��=k2��c2(NO2)����Ŀ�귴Ӧ2NO��g��+O2��g��2NO2��g������H=��+��=��H1+��H2���ɷ�Ӧ��ƽ��״̬������v1��=v1����v2��=v2��������v1����v2��=v1����v2������k1��c2��NO����k2��c��N2O2��c��O2��=k1��c��N2O2����k2��c2��NO2������K=
2NO2(g)(��) ��H2<0��v2��=k2��c2(N2O2)c(O2)��v2��=k2��c2(NO2)����Ŀ�귴Ӧ2NO��g��+O2��g��2NO2��g������H=��+��=��H1+��H2���ɷ�Ӧ��ƽ��״̬������v1��=v1����v2��=v2��������v1����v2��=v1����v2������k1��c2��NO����k2��c��N2O2��c��O2��=k1��c��N2O2����k2��c2��NO2������K=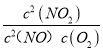 =
=![]() ���÷�ӦΪ���ȷ�Ӧ���¶����ߣ���Ӧ�ڵ�ƽ�����ƣ�k2����k2������a��b��2NO(g)+O2(g)
���÷�ӦΪ���ȷ�Ӧ���¶����ߣ���Ӧ�ڵ�ƽ�����ƣ�k2����k2������a��b��2NO(g)+O2(g) ![]() 2NO2(g)Ϊ���ȷ�Ӧ���¶����ߣ���Ӧ�����ڵ�ƽ������ƣ����ڷ�Ӧ�ٵ����ʴ���c(N2O2)��С����̶ȴ���k2����c(O2)����ij̶ȣ�ʹ���ߵij˻���v2����С���ʴ�Ϊ���������Խ��һ����ӳ�Ϊ�����Խ�ѣ���Ӧ����Խ����
2NO2(g)Ϊ���ȷ�Ӧ���¶����ߣ���Ӧ�����ڵ�ƽ������ƣ����ڷ�Ӧ�ٵ����ʴ���c(N2O2)��С����̶ȴ���k2����c(O2)����ij̶ȣ�ʹ���ߵij˻���v2����С���ʴ�Ϊ���������Խ��һ����ӳ�Ϊ�����Խ�ѣ���Ӧ����Խ���� ![]() �������¶����ߣ���Ӧ�����ڵ�ƽ������ƣ����ڷ�Ӧ�ٵ����ʴ���c(N2O2)��С����̶ȴ���k2����c(O2)����ij̶ȣ�ʹ���ߵij˻���v2����С��
�������¶����ߣ���Ӧ�����ڵ�ƽ������ƣ����ڷ�Ӧ�ٵ����ʴ���c(N2O2)��С����̶ȴ���k2����c(O2)����ij̶ȣ�ʹ���ߵij˻���v2����С��
��2���ٸ���ͼʾ��֪���ڴ����������£�C2H4��NO��O2��Ӧ��������N2��CO2��H2O����Ӧ��n(NO):n(O2) =2��1����Ӧ�ܷ���ʽΪ6NO+3O2+2C2H4![]() 3N2+4CO2+4H2O����ͼ��֪��b���ߵ���ߵ㴦�������ʸߣ������ʵͣ����˵��¶ȣ��ʺ�����Ϊ350�桢������3%���ʴ�Ϊ��6NO+3O2+2C2H4
3N2+4CO2+4H2O����ͼ��֪��b���ߵ���ߵ㴦�������ʸߣ������ʵͣ����˵��¶ȣ��ʺ�����Ϊ350�桢������3%���ʴ�Ϊ��6NO+3O2+2C2H4![]() 3N2+4CO2+4H2O��350�桢������3.0%��
3N2+4CO2+4H2O��350�桢������3.0%��
����NO��ֱ�Ӵ�NO�ֽ�����N2��O2����Ӧ���ܷ�ӦΪ2NO![]() O2+N2�����ݷ�Ӧԭ����Ni2+Ϊ���������ܷ�Ӧ-��2Ni2++2Vo+2NO��2Ni3++2O-+N2��-��2O-��+O2-+1/2O2+Vo)�ã�2Ni3+ + O2-��2Ni2+ +VO+
O2+N2�����ݷ�Ӧԭ����Ni2+Ϊ���������ܷ�Ӧ-��2Ni2++2Vo+2NO��2Ni3++2O-+N2��-��2O-��+O2-+1/2O2+Vo)�ã�2Ni3+ + O2-��2Ni2+ +VO+![]() O2���ʴ�Ϊ��2Ni3+ + O2-��2Ni2+ +VO+
O2���ʴ�Ϊ��2Ni3+ + O2-��2Ni2+ +VO+![]() O2��
O2��
��3�����NO�Ʊ�NH4NO3��������ӦΪNO-3e-+2H2O=NO3-+4H+��������ӦΪ��NO+5e-+6H+=NH4++H2O���ʴ�Ϊ��NO + 6H+ +5e- = NH4+ + H2O��

 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�����Ŀ���ס��ҡ��������������������У��ס��ҡ����о�����ij����ͬ��Ԫ�أ�����֮�������ͼ��ʾת����ϵ(��Ӧ���������ֲ�������ȥ)�������й����ʵ��ƶϲ���ȷ����(����)

ѡ�� | ���� | ���� |
A | ��ΪAl(OH)3 | ������������ |
B | ��ΪNa2CO3��Һ | �������CO2 |
C | ��ΪFe | ������������ |
D | ��ΪN2 | ����������� |
A. A B. B C. C D. D
����Ŀ��Ϊ�ᴿ�������ʣ�����������Ϊ���ʣ�����ѡ�õ��Լ��ͷ��뷽����ȷ���ǣ� ��
�� �� | �����Լ� | ���뷽�� | |
A | ����ͭ��Һ���������� | ͭ�� | �ᾧ |
B | Cl2��HCl�� | NaOH��Һ | ϴ�� |
C | Br2��H2O�� | �ƾ� | ��ȡ |
D | ͭ�ۣ����ۣ� | ϡ���� | ���� |
A.A
B.B
C.C
D.D