��Ŀ����
1���뽫������������б仯��������ڶ�Ӧ�ĺ����ϣ��ٵ������������������ˮ�����Ȼ�������ˮ�����ռ��ۻ������Ȼ�������ˮ�����Ȼ�����ȷֽ⣮��1����ѧ��û�б��ƻ����Ǣ٢ڣ�
��2�����������Ӽ��ƻ����Ǣۢܣ�
��3�����������ۼ��ƻ����Ǣݣ�
��4���ȷ������Ӽ��ַ������ۼ��ƻ����Ǣޣ�
��5����֪��1mol H-H����1mol N��N��1mol N-H���ֱ���Ҫ���յ�����Ϊ436kJ��946k J��391k J������N2��H2��Ӧ����1mol NH3��Ҫ�ų�46kJ��������
���� һ����˵�����ý�����ǽ����γ����Ӽ����ǽ���֮���γɹ��ۼ�����ѧ�仯�С���������о�������ѧ���Ķ��ѣ����ɷ��ӹ��ɵ����ʵ���̬�仯���ǵ���ʵ��ܽ��л�ѧ�����䣬��H=���ѻ�ѧ�����յ�����-���ɻ�ѧ���ͷŵ��������Դ������
��� �⣺�ٵ���������������仯��ֻ��״̬�����仯��ֻ�ƻ����Ӽ���������û�л�ѧ�����ƻ���
����������ˮ��ֻ�ƻ����Ӽ�����������ѧ�����䣻
���Ȼ�������ˮ����ˮ���ӵ������£��Ȼ����е����Ӽ����ƻ��������ƻ��������Ӽ���
���ռ��ۻ��д������Ӽ����ڻ��ռ�ʱ���������Ӽ�Ļ�ѧ�����ƻ��������ƻ��������Ӽ���
���Ȼ�������ˮ����ˮ���ӵ������£��Ȼ����еĹ��ۼ����ƻ��������ƻ����ǹ��ۼ���
���Ȼ�����ȷֽ⣬�Ȼ�������ӻ�������ڵĻ�ѧ�������Ӽ������ۼ������ȷֽ�ʱ���������Ӽ�Ļ�ѧ������笠������еĹ��ۼ����ƻ������������ƻ��������Ӽ������ۼ���
��1����ѧ��û�б��ƻ����Ǣ٢ڣ��ʴ�Ϊ���٢ڣ�
��2�����������Ӽ��ƻ����Ǣۢܣ��ʴ�Ϊ���ۢܣ�
��3�����������ۼ��ƻ����Ǣݣ��ʴ�Ϊ���ݣ�
��4���ȷ������Ӽ��ַ������ۼ��ƻ����Ǣޣ��ʴ�Ϊ���ޣ�
��5����1molH-H����1molN��N��1molN-H���ֱ���Ҫ���յ�����Ϊ436kJ��946kJ��391kJ���ڷ�ӦN2+3H2?2NH3�У�����3mol H-H����1mol N��N�������յ�����Ϊ��3��436kJ+946kJ=2254kJ��
����2mol NH3�����γ�6molN-H�����ų�������Ϊ��6��391kJ=2346kJ��
���յ������٣��ų��������࣬�÷�ӦΪ���ȷ�Ӧ��
�ų�������Ϊ��2346kJ-2254kJ=92kJ��
��������1mol NH3�ų�����Ϊ46kJ��
�ʴ�Ϊ��46 kJ��
���� ���⿼�黯ѧ������Ӧ�������仯��Ϊ��Ƶ���㣬���ջ�ѧ�����γɼ���Ӧ�ȵļ����Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | 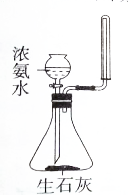 ��ȡ�������� | B�� | 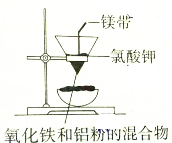 ���ƵĽ����� | ||
| C�� |  ���������� | D�� | 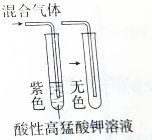 ��֤���������һ��������ϩ |
| A�� | CH4+2O2$\stackrel{��ȼ}{��}$CO2+2H2O | B�� | 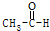 +H2$\stackrel{����}{��}$CH3CH2OH +H2$\stackrel{����}{��}$CH3CH2OH | ||
| C�� | H2+Cl2�T2HCl | D�� | 2CH3CH2OH+O2��2CH3CHO+2H2O |
| A�� | ���������ϩ�Ļ������ͨ�����Ը��������Һ���ɳ�ȥ��ϩ | |
| B�� | ����������Һ����һ����AgNO3��Һ�У���εμ�ϡ��ˮ������ǡ���ܽ�Ϊֹ | |
| C�� | ��֤RXΪ����飬��RX���ռ�ˮ��Һ��ϼ��ȣ�����Һ��ȴ���ټ�����������Һ | |
| D�� | ��ˮ�Ҵ���Ũ���Ṳ����170�棬���Ƶõ�����ͨ�����Ը�����ؿɼ����Ƶõ������Ƿ�Ϊ��ϩ |
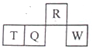 ������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������T������������������������ȣ������жϲ���ȷ���ǣ�������?
������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������T������������������������ȣ������жϲ���ȷ���ǣ�������?| A�� | �����̬�⻯������ȶ��ԣ�R��Q | |
| B�� | ����������Ӧˮ��������ԣ�Q��W? | |
| C�� | ԭ�Ӱ뾶��T��Q��R | |
| D�� | T������������Ӧˮ����Ϊǿ�� |
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | V��A | O |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� | �� | ⑪ | ⑫ |
��2����ЩԪ�ص����������Ķ�Ӧˮ������HClO4������ǿ��NaOH������ǿ��
��3���ݡ��ߵ����������Ķ�Ӧˮ�������Ӧ�Ļ�ѧ����ʽAl��OH��3+NaOH�TNaAlO2+2H2O
��4�����γɵ��ʵĽṹʽN��N���ٺ͢��γɵ�����������ĵ���ʽ
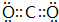 ��
����5������ЩԪ���У�ԭ�Ӱ뾶���ķǽ���Ԫ�ص�ԭ����Si��
| A�� | Ŀǰ��ѧ���Ѿ��Ƶõ�ԭ�Ӳ��࣬�����Ǩ�����ǹ��10��������ȡ��������������õľ���� | |
| B�� | ʯ���ѽ����ҪĿ����������͵������͵IJ�����������ʯ�ʹ��ѻ�����ҪĿ���ǵõ��������ϩ����ϩ����̬������ | |
| C�� | �Ʋ����������Ƭ�ȶ�п��Ƭ����ʴ | |
| D�� | ���������ǹ��ұ����İ���������ʼ�������֮�ƣ���֪�ò����Ի�ѧҩƷ���У��Ҵ�������������Һ�����Խ������������ﵽ������Ŀ�� |
| A�� |  ��ͼװ�ÿ������Ʊ��������� | |
| B�� | 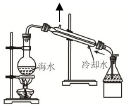 ��ͼװ�ÿ�����ģ�⺣ˮ���� | |
| C�� | 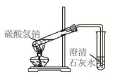 ��ͼװ�ÿ�����̽��̼�����Ƶ����ȶ��� | |
| D�� | 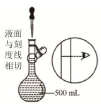 ��ͼװ��Ϊ������Һ�����еĶ��ݲ��� |