��Ŀ����
����Ŀ����1����ͬ��ͬѹ�£�2gCO2�����Ϊ1120mL����2gA��������Ϊ770mL��������A��Ħ������Ϊ__________mol/L��
��2������A��B��C���ֻ������ȡ40g���ϣ���ȫ��Ӧ��B 18g��C49g������D���ɡ���֪D��ʽ��Ϊ106���ֽ�22gA��11gB��Ϸ�Ӧ��������D_____ mol��
��3����������李�NH4Al(SO4)2����Һ�еμ�Ba(OH)2��Һ�����ȣ��պ�ʹNH4+ȫ��ת��ΪNH3��д����Ӧ����������ʽ��________________________________________��
���𰸡� 64 0.25 NH4++Al3++2SO42��+2Ba2++4OH�� ![]() NH3��+Al��OH��3��+2BaSO4��+H2O
NH3��+Al��OH��3��+2BaSO4��+H2O
����������1�����ݰ���٤�����ɵ����ۣ�ͬ��ͬѹ�£�ͬ���������壬���ʵ���֮��=���֮��=Ħ�������ĵ����ȣ���M(A)��M(CO2)=V(CO2)��V(A)������M(A)= ![]() = 64g/mol��
= 64g/mol��
��2�������⣬40gA��ȫ��Ӧ����Ӧ��B������Ϊ40g-18g=22g�����ɵ�C������Ϊ49g-40g=9g�����������غ㶨�ɣ����ɵ�D������Ϊ40g+22g-9g=53g��11gB��ȫ��Ӧ��ҪA������Ϊ40g��![]() =20g����22gA��11gB��Ӧ��A��ʣ�࣬B��ȫ��Ӧ����������D������Ϊ53g��
=20g����22gA��11gB��Ӧ��A��ʣ�࣬B��ȫ��Ӧ����������D������Ϊ53g��![]() =26.5g�����ʵ���Ϊ��26.5g��106g/mol=0.25mol��
=26.5g�����ʵ���Ϊ��26.5g��106g/mol=0.25mol��
��3�����������[NH4Al(SO4)2]��Һ�еμ�Ba(OH)2��Һ�����ȣ�������Al(OH)3������������NH3H2O�����պ�ʹNH4+ȫ��ת��ΪNH3�����������[NH4Al(SO4)2]��Ba(OH)2Ӧ�����ʵ���֮��1��2��Ӧ�����ӷ���ʽΪ��NH4++Al3++2SO42-+2Ba2++4OH-![]() NH3��+Al(OH)3��+2BaSO4��+H2O��
NH3��+Al(OH)3��+2BaSO4��+H2O��

����Ŀ��1��2-��������������Ϳ����������Ӽ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1��2-�������飮����A��F��װ���Ҵ���Ũ����Ļ��Һ��D�е��Թ���װ��Һ�壮���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������l40����ˮ�������ѡ����г�װ������ȥ��
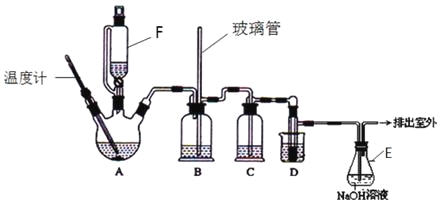
�й������б����£�
�Ҵ� | 1,2-�������� | ���� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
�ܶȣ�g�� cm-3 | 0.79 | 2.2 | 0.71 |
�е㣯�� | 78.5 | 132 | 34.6 |
�۵㣯�� | һl30 | 9 | -1l6 |
��д���пհף�
��1��A����Ҫ���������Ҵ�����ˮ��Ӧ������ȥ��Ӧ������д���Ҵ��������ȥ��Ӧ�Ļ�ѧ����ʽ�� ��
D�з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
��2����ȫƿB���Է�ֹ�����������Լ��ʵ�����ʱ�����Ƿ�����������д����������ʱƿB�е����� ��
��3����װ��C��Ӧ���� ����Ŀ�������շ�Ӧ�п������ɵ��������壺������ȷѡ��ǰ����ĸ��
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��4������E��NaOH��Һ�������� ��
��5�������������������������ѣ�����________����������ƣ��ķ�����ȥ��
��6����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ������ϩ���巴Ӧʱ���ȣ���ȴ�ɱ�����Ĵ����ӷ������ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ����_____________��

