��Ŀ����
4��ijʵ������Ҫ����240mL 0.2mol��L-1��Na2CO3��Һ���ش��������⣺��1��ʹ��������ƽ����Na2CO3•10H2O������Ϊ14.3 g��
��2������������Һʱ�������õ��ձ���������������ʹ�õIJ���������250mL����ƿ����ͷ�ι�
��3�����в�����ʹ������ҺŨ��ƫ�ߵ���AC��
A����ѡ�õ������Ѿ����ʣ����ֵ�ʧȥ�˽ᾧˮ
B��ת��ǰ������ƿ�к�����������ˮ
C����δ��ȴ����Һ�ز�����ע������ƿ��
D������ʱ�����ӿ̶���
E������ʱ�����Ϊ���������
F�����ݺ�ʹ��Һ���ȣ���ֹ�����ְ�Һ����ڿ̶��ߣ�����������ˮ���̶���
��4��ȡ�����Ƶ�̼������Һ125mL���������125mL 0.3mol��L-1��������Һ������ַ�Ӧ�����ɵ������ڱ�״������ռ�����Ϊ���������������ȫ���ݳ���0.56L���跴Ӧ����Һ�����Ϊ250mL����Ӧ����Һ��Na+�����ʵ���Ũ��Ϊ0.2mol/L��
���� ��1������n=cv��������Na2CO3�����ʵ���������Na2CO3•10H2O�����ʵ�������Na2CO3�����ʵ���������m=nM����Na2CO3•10H2O��������
��2������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��3������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��4�����������̼������ȫ��Ӧ���ɶ�����̼������n=cV����̼���Ƶ����ʵ���������̼ԭ���غ���������̼�����ʵ������ٸ���V=nVm���������̼�����������c=$\frac{n}{V}$���㷴Ӧ�������ӵ�Ũ�ȣ�
��� �⣺��1����������Һ�����Ϊ240ml��������ƿ�Ĺ��û��240ml��ֻ��ѡ��250ml��Na2CO3�����ʵ���n=cV=0.25L��0.2mol•L-1=0.05mol��Na2CO3•10H2O�����ʵ�������Na2CO3�����ʵ���������Na2CO3•10H2O������0.05mol��286g/mol=14.3g���ʴ�Ϊ��14.3��
��2�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������250mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��3��A����ѡ�õ������Ѿ����ʣ����ֵ�ʧȥ�˽ᾧˮ�����ʹҩƷ�е�Na2CO3������ƫ����Ũ��ƫ�ߣ���A��ȷ��
B��ת��ǰ������ƿ�к�����������ˮ����������Һ��Ũ����Ӱ�죬��B����
C����δ��ȴ����Һ�ز�����ע������ƿ�У�����ȴ����Һ���ƫС����Ũ��ƫ�ߣ���C��ȷ��
D������ʱ�����ӿ̶��ߣ�����Һ���ƫ��Ũ��ƫ�ͣ���D����
E������ʱ�����Ϊ���������������������ҩƷ������ƫС��Ũ��ƫ�ͣ���E����
F�����ݺ�ʹ��Һ���ȣ���ֹ�����ְ�Һ����ڿ̶����������ģ�����������ˮ���̶��ߣ�����ҺŨ��ƫ�ͣ���F����
�ʴ�Ϊ��AC��
��4��125mL0.2mol/LNa2CO3��Һ��̼���Ƶ����ʵ���=0.125L��0.2mol/L=0.025mol��125mL 0.3mol•L-1 ��������Һ����������ʵ���=0.125L��0.3mol/L=0.0375mol�������������̼������ȫ��Ӧ���ɶ�����̼����̼ԭ���غ��֪������̼�����ʵ���Ϊ0.025mol�����Ϊ0.025mol��22.4L/mol=0.56L��
��Ӧ����Һ�����������ʵ������䣬Ϊ0.025mol��2=0.05mol���������ӵ����ʵ���Ũ��=$\frac{0.05mol}{0.25L}$=0.2mol/L��
�ʴ�Ϊ��0.56L��0.2mol/L��
���� ���⿼��һ�����ʵ���Ũ����Һ���ơ���ѧ����ȣ��ѶȲ���ע�����֪ʶ���������գ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �����Һ���ȼ������ᱵ��Һ�а�ɫ�������ټ���ϡ���ᣬ��ɫ��������ʧ������ȷ�ϴ���Һ�к���SO42- | |
| B�� | �����Һ�м���NaOH��Һ�����ȣ�������������ʹʪ��ĺ�ɫʯ����ֽ����������ȷ�ϴ���Һ�к���NH4+ | |
| C�� | �����Һ���ȼ�����ˮ���ٵ���KSCN��Һ����Һ��죬����ȷ�ϴ���Һ�к���Fe2+ | |
| D�� | �ò�����պȡ����Һ���ھƾ��ƻ��������գ�����ʻ�ɫ������ȷ�ϴ���Һ�к���Na+ |
| A�� | 1 mol H2������ֻ���ڱ�״���²�ԼΪ2g | |
| B�� | �ڱ�״����ij����������22.4L������Ϊ����������ʵ���Լ��1mol | |
| C�� | ��20��ʱ��1mol�κ����������ܱ�22.4L�� | |
| D�� | 1mol H2��O2�Ļ���������������Ϊ34g |
| A�� | +7 | B�� | +2 | C�� | +3 | D�� | +4 |
| A�� | ��������������һ�� | B�� | �����Ũ������һ������������ | ||
| C�� | �¶Ƚ��͵�25�� | D�� | ����KHCO3��ĩ���� |

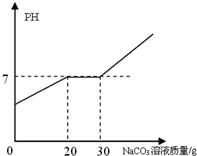 ��21.88g ���������Ȼ��Ƶ�ϡ�����м���10.6%��̼������Һ���������̼������Һ��������ҺPH�Ĺ�ϵ��ͼ��ʾ��
��21.88g ���������Ȼ��Ƶ�ϡ�����м���10.6%��̼������Һ���������̼������Һ��������ҺPH�Ĺ�ϵ��ͼ��ʾ��