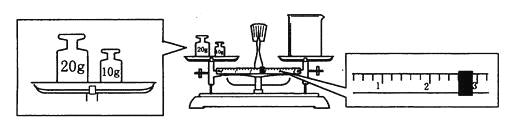
��Ŀ����
����Ŀ������Բ����ڷ�����������ͨ��������й㷺��Ӧ��ǰ������ͼ��һ�����ô���ʯ(��Ҫ�ɷ�CaCO3��MgCO3)��������(��Ҫ�ɷ�Al2O3����������Fe2O3����)�Ʊ�һ�ֳ���Բ���(Ca12Al14Ox)�Ĺ�������ͼ���ش��������⣺
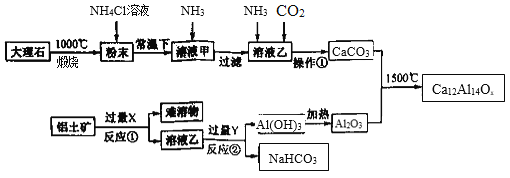
(1)Ca12Al14Ox��x����ֵΪ ______���Լ�X��������_________��
(2)NH4Cl��Һ���ܽ�����ĩ����ԭ����_________����������Ҫ��Ӧ�Ļ�ѧ����Ϊ______��
(3)��Ӧ�ڵ����ӷ���ʽΪ _______��
(4)ʵ���Ҽ���Al(OH)3Ӧ��________(����������)���У������ٰ���_____������ȡ�
(5)����Һ����c(Mg2+) <5��10-5molL-1Ԫ����ʧ����ʱӦ������ҺpH����______��Ϊ���ٸ�Ԫ����ʧ��Ӧ������Һ����c(Ca2+)��______��������Ksp[Mg(OH)2]=5��10��11��Ksp[Ca(OH)2]=5��10��6��
���𰸡�33 ����������Һ NH4Cl��Һ��笠�����ˮ�⣬��Һ������ CaCl2��2NH3��CO2��H2O=CaCO3����2NH4Cl AlO2����CO2��2H2O=Al(OH)3����HCO3�� ���� ���ˡ�ϴ�� 11 5mol��L��1
��������
����ʯ�ڸ��������գ�CaCO3��MgCO3���ֽ�����CaO��MgO������NH4Cl�ܽ⣬�õ�CaCl2��MgCl2�Ļ����Һ��ͨ��NH3��Mg2��ת��ΪMg(OH)2�����˳�ȥ��CaCl2��Һ�м���NH3��CO2�õ�CaCO3���������к���Al2O3��Fe2O3������Al2O3����ǿ����з��룬����ǿ��õ�AlO2����Һ��ͨ��CO2���ɽ�AlO2��ת��ΪAl(OH)3�����ȷֽ�õ�Al2O3�����յõ�����Բ��ϡ�
(1)���ݻ������и���Ԫ�ػ��ϼ۵Ĵ����͵����㣬Ca12Al14Ox��Ca��Al�Ļ��ϼ۷ֱ�Ϊ��2����3������12��2��14��3��(��2)x=0����x=33��
�������к���Al2O3��Fe2O3������Al2O3����ǿ����з��룬XΪǿ����ݺ�������̵õ�NaHCO3����֪��ǿ��Ϊ����������Һ��
(2)CaCO3��MgCO3�ڸ�������������CaO��MgO��ĩ����NH4ClΪǿ�������Σ�����Һ�е�NH4��ˮ��ʹ����Һ�����ԣ��ܹ��ܽ�CaO��MgO������Ca2����Mg2����ͨ��NH3��Mg2���γ�Mg(OH)2���������˳�ȥ����Һ��ΪCaCl2��Һ����ͨ��NH3��CO2��������ӦCaCl2��2NH3��CO2��H2O=CaCO3����2NH4Cl��
(3)������ͼ��֪����Ӧ��ΪNaAlO2��Һ��ͨ�������CO2������Al(OH)3��NaHCO3�����ӷ���ʽΪAlO2����CO2��2H2O=Al(OH)3����HCO3����
(4)���ȹ�����Ҫ��������������Ϊ�˵õ�CaCO3�������������Ϊ���ˡ�ϴ�ӡ�����ȣ�
(5)![]() =5��10��11������Һ����c(Mg2+)<5��10-5molL-1���������ݣ�
=5��10��11������Һ����c(Mg2+)<5��10-5molL-1���������ݣ�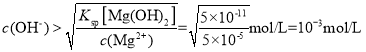 ����pH=
����pH=![]() ����pHӦ�ô���11��
����pHӦ�ô���11��
pH=11ʱ����Һ��c(OH��)=10��3mol��L��1���������ݣ�![]() ���������ݣ�c(Ca2��)=5mol��L��1��Ϊ���ٸ�Ԫ����ʧ��Ӧ������Һ����c(Ca2+)��5mol��L��1��
���������ݣ�c(Ca2��)=5mol��L��1��Ϊ���ٸ�Ԫ����ʧ��Ӧ������Һ����c(Ca2+)��5mol��L��1��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�