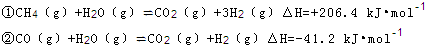��Ŀ����
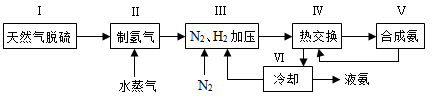
��14�֣��״��������ѵȱ���Ϊ��ɫ��Դ����ҵ��������Ȼ��Ϊ��Ҫԭ���������̼��ˮ������һ���������Ʊ��ϳ�����CO��H2�������Ƴɼ״��������ѣ�CH3OCH3����
��1����֪1g������������ȫȼ�������ȶ���������ų�������Ϊ32kJ����д��������ȼ���ȵ��Ȼ�ѧ����ʽ____________________________________________________________________��
��2��д�������Ѽ���ȼ�ϵ�صĸ����缫��Ӧʽ __________________________________��
��3���úϳ����Ʊ������ѵķ�Ӧԭ��Ϊ��2CO(g) + 4H2(g) CH3OCH3(g) + H2O(g)����֪һ�������£��÷�Ӧ��CO��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO)]�ı仯����������ͼ��
CH3OCH3(g) + H2O(g)����֪һ�������£��÷�Ӧ��CO��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO)]�ı仯����������ͼ��
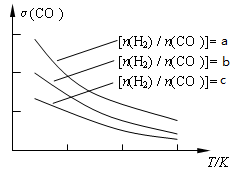
��a��b��c���Ӵ�С��˳������Ϊ_________________���÷�Ӧ�ġ�H_______0�������������������
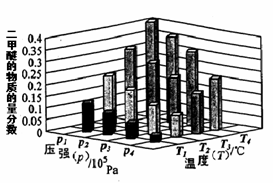
��ij�¶��£���2.0molCO(g)��4.0molH2(g)�����ݻ�Ϊ2L���ܱ������У���Ӧ����ƽ��ʱ���ı�ѹǿ���¶ȣ�ƽ����ϵ��CH3OCH3(g)�����ʵ��������仯�������ͼ��ʾ�������¶Ⱥ�ѹǿ�Ĺ�ϵ�ж���ȷ���� ��
A. P3��P2��T3��T2 B. P1��P3��T1��T3 C. P2��P4��T4��T2 D. P1��P4��T2��T3
���ں����ܱ������ﰴ�����Ϊ1:2����һ����̼��������һ�������·�Ӧ�ﵽƽ��״̬�����ı䷴Ӧ��ijһ�����������б仯��˵��ƽ��һ�����淴Ӧ�����ƶ����� ��
A. ����Ӧ������������С B. �淴Ӧ������������С
C. ��ѧƽ�ⳣ��Kֵ��С D. ������ת���ʼ�С
�� ij�¶��£���4.0molCO��8.0molH2�����ݻ�Ϊ2L���ܱ������У���Ӧ�ﵽƽ��ʱ����ö����ѵ��������Ϊ25%������¶��·�Ӧ��ƽ�ⳣ��K��__________��
��1����֪1g������������ȫȼ�������ȶ���������ų�������Ϊ32kJ����д��������ȼ���ȵ��Ȼ�ѧ����ʽ____________________________________________________________________��
��2��д�������Ѽ���ȼ�ϵ�صĸ����缫��Ӧʽ __________________________________��
��3���úϳ����Ʊ������ѵķ�Ӧԭ��Ϊ��2CO(g) + 4H2(g)
 CH3OCH3(g) + H2O(g)����֪һ�������£��÷�Ӧ��CO��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO)]�ı仯����������ͼ��
CH3OCH3(g) + H2O(g)����֪һ�������£��÷�Ӧ��CO��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2) / n(CO)]�ı仯����������ͼ��

��a��b��c���Ӵ�С��˳������Ϊ_________________���÷�Ӧ�ġ�H_______0�������������������
��ij�¶��£���2.0molCO(g)��4.0molH2(g)�����ݻ�Ϊ2L���ܱ������У���Ӧ����ƽ��ʱ���ı�ѹǿ���¶ȣ�ƽ����ϵ��CH3OCH3(g)�����ʵ��������仯�������ͼ��ʾ�������¶Ⱥ�ѹǿ�Ĺ�ϵ�ж���ȷ���� ��
A. P3��P2��T3��T2 B. P1��P3��T1��T3 C. P2��P4��T4��T2 D. P1��P4��T2��T3
���ں����ܱ������ﰴ�����Ϊ1:2����һ����̼��������һ�������·�Ӧ�ﵽƽ��״̬�����ı䷴Ӧ��ijһ�����������б仯��˵��ƽ��һ�����淴Ӧ�����ƶ����� ��
A. ����Ӧ������������С B. �淴Ӧ������������С
C. ��ѧƽ�ⳣ��Kֵ��С D. ������ת���ʼ�С
�� ij�¶��£���4.0molCO��8.0molH2�����ݻ�Ϊ2L���ܱ������У���Ӧ�ﵽƽ��ʱ����ö����ѵ��������Ϊ25%������¶��·�Ӧ��ƽ�ⳣ��K��__________��
��1��CH3OCH3(g) + 3O2(g)��2CO2(g) + 3H2O(l) ��H��-1472kJ/mol
��2��CH3OCH3 -12e- + 16OH-��2CO32- + 11H2O
��3����a��b��c���� ��B��D ��B��C �� 2.25
��2��CH3OCH3 -12e- + 16OH-��2CO32- + 11H2O
��3����a��b��c���� ��B��D ��B��C �� 2.25
�����������1��1g������������ȫȼ�������ȶ���������ų�������Ϊ32kJ����1mol�����Ѽ�46g��������ȫȼ�������ȶ���������ų�������Ϊ46��32kJ��1472kJ����˶�����ȼ���ȵ��Ȼ�ѧ����ʽΪCH3OCH3(g) + 3O2(g)��2CO2(g) + 3H2O(l) ��H��-1472kJ/mol��
��2��ԭ����и���ʧȥ���ӣ���˶������ڸ���ͨ�룬����������Ӧ����������Լ��ԣ�������ѵ������������ձ�Ϊ̼���Σ���˶����Ѽ���ȼ�ϵ�صĸ����缫��ӦʽΪCH3OCH3 -12e- + 16OH-��2CO32- + 11H2O��
��3����������CO���Ƿ�Ӧ����Ͷ�ϱ�[n(H2) / n(CO)]Խ��CO��ת����Խ�����Ը���ͼ���֪a��b��c��ͬ������ͼ���֪�����¶�CO��ת���ʽ��ͣ���˵�������¶�ƽ�����淴Ӧ�����ƶ����������Ӧ�Ƿ��ȷ�Ӧ������H��0��
�ڸ���ͼ����Կ��������ڷ�Ӧ��2CO(g) + 4H2(g)
 CH3OCH3(g) + H2O(g) ��H��0��ѹǿԽ�����ѵ����ʵ���������Խ���¶�Խ�ߣ������ѵ����ʵ�������ԽС������P1��P3��T1��T3��P1��P4��T2��T3���ʴ�Ϊ��BD��
CH3OCH3(g) + H2O(g) ��H��0��ѹǿԽ�����ѵ����ʵ���������Խ���¶�Խ�ߣ������ѵ����ʵ�������ԽС������P1��P3��T1��T3��P1��P4��T2��T3���ʴ�Ϊ��BD���ۻ�ѧƽ��ı�־�����淴Ӧ������ȣ����淴Ӧ���ʴ�������Ӧ����ʱ��˵����Ӧ��������еġ�A������Ӧ������������С��˵��ƽ��������Ӧ�����ƶ���A����ȷ��B���淴Ӧ������������С��˵��ƽ�����淴Ӧ�����ƶ���B��ȷ��C����ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ����˻�ѧƽ�ⳣ��Kֵ��С��˵��ƽ�����淴Ӧ�����ƶ���C��ȷ��D��������ת���ʼ�С�����ж�ƽ���ƶ�����D����ȷ���ʴ�Ϊ��BC��
�ܸ��ݷ�Ӧ�Ļ�ѧ����ʽ��֪��
2CO(g) + 4H2(g)
 CH3OCH3(g) + H2O(g)
CH3OCH3(g) + H2O(g)��ʼŨ�ȣ�mol/L�� 2 4 0 0
ת��Ũ�ȣ�mol/L�� 2x 4x x x
ƽ��Ũ�ȣ�mol/L��2��2x 4��4x x x
��Ӧ�ﵽƽ��ʱ����ö����ѵ��������Ϊ25%
��
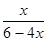 ��0.25
��0.25���x��0.75
���Ը��¶���ƽ�ⳣ��K��
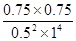 ��2.25
��2.25
��ϰ��ϵ�д�
�����Ŀ
 C(g)����һʱ�̵������淴Ӧ���ʿ��æ�����������ʾ��
C(g)����һʱ�̵������淴Ӧ���ʿ��æ�����������ʾ��

 ������Ӧ���ȣ������n��O2����ʱ��ı仯���±�
������Ӧ���ȣ������n��O2����ʱ��ı仯���±�
 2Z(g) ��H��0��һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ���������±���
2Z(g) ��H��0��һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ���������±���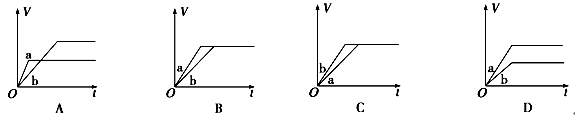
 2NH3��g�� ?H=��92.4kJ?mol?1
2NH3��g�� ?H=��92.4kJ?mol?1