ƒøƒ⁄»ð
£®10∑÷£©Ω´“ª∂®¡øµƒSO2∫Õ∫¨0.7mol—ı∆¯µƒø’∆¯(∫ˆ¬‘CO2)∑≈»Î0.5 L√б’»ð∆˜ƒ⁄£¨550°Ê ±£¨‘⁄¥þªØº¡◊˜”√œ¬∑¢…˙∑¥”¶£∫ £®’˝∑¥”¶∑≈»»£©°£≤‚µ√n£®O2£©ÀÊ ±º‰µƒ±‰ªØ»Áœ¬±Ì
£®’˝∑¥”¶∑≈»»£©°£≤‚µ√n£®O2£©ÀÊ ±º‰µƒ±‰ªØ»Áœ¬±Ì

∑¥”¶¥ÔµΩ5s∫Û£¨Ω´»ð∆˜÷–µƒªÏ∫œ∆¯ÃÂÕ®π˝π˝¡øNaOH»Ð“∫£¨∆¯ÃÂê˝ºı…Ÿ¡À22. 4L£®¥Àê˝Œ™±Í◊º◊¥øˆœ¬µƒÃª˝£©£ª‘ŸΩ´ £”ý∆¯ÃÂÕ®π˝Ωπ–‘√ª ≥◊”À·µƒºÓ–‘»Ð“∫Œ¸ ’O2£¨∆¯ÃµƒÃª˝”÷ºı…Ÿ¡À5.6L£®¥Àê˝Œ™±Í◊º◊¥øˆœ¬µƒÃª˝£©°£
«Îªÿ¥œ¬¡–Œ £∫
£®1£©”√O2±Ì 楔0-lsƒ⁄∏√∑¥”¶µƒ∆Ωæ˘∑¥”¶ÀŸ¬ Œ™__________________°£
£®2£©O2µƒ∆Ω∫‚≈®∂»c (O2)=____________________________£ª
£®3£© 4s ±£¨SO2µƒ…˙≥…ÀŸ¬ ____________£®ÃÓ°∞¥Û”⁄°±°¢°∞–°”⁄°±ªÚ°∞µ»”⁄°±£©O2µƒœ˚∫ƒÀŸ¬ °£
£®4£©«Û∏√∑¥”¶¥ÔµΩ∆Ω∫‚ ±SO2µƒ◊™ªØ¬ «________£®”√∞Ÿ∑÷ ˝±Ì 棩°£
£®5£©»ÙΩ´∆Ω∫‚ªÏî∆¯ÃÂ÷–SO3µƒ5%Õ®»Îπ˝¡øµƒBaCl2»Ð“∫£¨…˙≥…≥¡µÌ_______øÀ£®º∆À„Ω·π˚±£¡Ù“ªŒª–° ˝£©°£
 £®’˝∑¥”¶∑≈»»£©°£≤‚µ√n£®O2£©ÀÊ ±º‰µƒ±‰ªØ»Áœ¬±Ì
£®’˝∑¥”¶∑≈»»£©°£≤‚µ√n£®O2£©ÀÊ ±º‰µƒ±‰ªØ»Áœ¬±Ì
∑¥”¶¥ÔµΩ5s∫Û£¨Ω´»ð∆˜÷–µƒªÏ∫œ∆¯ÃÂÕ®π˝π˝¡øNaOH»Ð“∫£¨∆¯ÃÂê˝ºı…Ÿ¡À22. 4L£®¥Àê˝Œ™±Í◊º◊¥øˆœ¬µƒÃª˝£©£ª‘ŸΩ´ £”ý∆¯ÃÂÕ®π˝Ωπ–‘√ª ≥◊”À·µƒºÓ–‘»Ð“∫Œ¸ ’O2£¨∆¯ÃµƒÃª˝”÷ºı…Ÿ¡À5.6L£®¥Àê˝Œ™±Í◊º◊¥øˆœ¬µƒÃª˝£©°£
«Îªÿ¥œ¬¡–Œ £∫
£®1£©”√O2±Ì 楔0-lsƒ⁄∏√∑¥”¶µƒ∆Ωæ˘∑¥”¶ÀŸ¬ Œ™__________________°£
£®2£©O2µƒ∆Ω∫‚≈®∂»c (O2)=____________________________£ª
£®3£© 4s ±£¨SO2µƒ…˙≥…ÀŸ¬ ____________£®ÃÓ°∞¥Û”⁄°±°¢°∞–°”⁄°±ªÚ°∞µ»”⁄°±£©O2µƒœ˚∫ƒÀŸ¬ °£
£®4£©«Û∏√∑¥”¶¥ÔµΩ∆Ω∫‚ ±SO2µƒ◊™ªØ¬ «________£®”√∞Ÿ∑÷ ˝±Ì 棩°£
£®5£©»ÙΩ´∆Ω∫‚ªÏî∆¯ÃÂ÷–SO3µƒ5%Õ®»Îπ˝¡øµƒBaCl2»Ð“∫£¨…˙≥…≥¡µÌ_______øÀ£®º∆À„Ω·π˚±£¡Ù“ªŒª–° ˝£©°£
£®1£©0.6mol/(L°§s)
£®2£©0.5mol/L
£®3£©¥Û”⁄
£®4£©90%
£®5£©10.5
£®2£©0.5mol/L
£®3£©¥Û”⁄
£®4£©90%
£®5£©10.5
‘Â∑÷Œˆ£∫£®1£©0-lsƒ⁄O2µƒŒÔ÷ µƒ¡øºı…Ÿ0.7-0.4=0.3mol£¨≈®∂»ºı…Ÿ0.3mol/0.5L=0.6mol/L£¨À˘“‘”√O2±Ì 楔0-lsƒ⁄∏√∑¥”¶µƒ∆Ωæ˘∑¥”¶ÀŸ¬ Œ™0.6mol/L/1s=0.6mol/(L°§s)
£®2£©∏˘æð“‚ø…÷™£¨”Ϋ‚—ıªØƒ∆µƒ∑¥”¶µƒ «∂˛—ıªØ¡Ú°¢»˝—ıªØ¡ÚµƒªÏ∫œ∆¯Ã£¨∂˛’þµƒŒÔ÷ µƒ¡ø÷Æ∫Õ”Îø™ ºº”»Îµƒ∂˛—ıªØ¡ÚµƒŒÔ÷ µƒ¡øœýÕ¨£¨À˘“‘ªÏ∫œ∆¯ÃÂÕ®π˝«‚—ıªØƒ∆»Ð“∫ê˝ºı…Ÿ£®±Í◊º◊¥øˆ£©22.4L£¨Àµ√˜ø™ ºÕ®»Îµƒ∂˛—ıªØ¡ÚµƒŒÔ÷ µƒ¡ø «1mol£¨ £”ý∆¯ÃÂÕ®π˝Ωπ–‘√ª ≥◊”À·µƒºÓ–‘»Ð“∫Œ¸ ’O2£¨∆¯ÃµƒÃª˝”÷ºı…Ÿ¡À5.6L£®±Í◊º◊¥øˆ£©£¨Àµ√˜ £”ý—ı∆¯5.6L£¨ŒÔ÷ µƒ¡ø «0.25mol£¨≈®∂» «5.6L/22.4L/mol/0.5L=0.5mol/L£¨À˘“‘—π«øµƒ∆Ω∫‚≈®∂» «0.5mol/L£ª
£®3£©4s ±£¨∑¥”¶“—¥Ô∆Ω∫‚£¨vƒÊ(SO2)=2v’˝£®O2£©£¨À˘“‘SO2µƒ…˙≥…ÀŸ¬ ¥Û”⁄O2µƒœ˚∫ƒÀŸ¬ °£
£®4£©—ı∆¯µƒ∆Ω∫‚ŒÔ÷ µƒ¡ø «0.25mol£¨‘Úœ˚∫ƒ—ı∆¯0.7mol-0.25mol=0.45mol£¨À˘“‘œ˚∫ƒ∂˛—ıªØ¡Ú0.45mol°¡2=0.9mol£¨∏˘æð£®2£©µƒ∑÷Œˆø…÷™£¨∂˛—ıªØ¡Úµƒ≥ı º¡ø «1mol£¨À˘“‘∏√∑¥”¶¥ÔµΩ∆Ω∫‚ ±SO2µƒ◊™ªØ¬ «0.9mol/1mol°¡100%=90%£ª
£®5£©ªÏ∫œ∆¯ÃÂ÷–µƒ»˝—ıªØ¡Ú”ÎBaCl2»Ð“∫∑¥”¶…˙≥…¡ÚÀ·±µ≥¡µÌ°£Õ¨¿Ìø…º∆À„≥ˆ…˙≥…»˝—ıªØ¡ÚµƒŒÔ÷ µƒ¡ø «0.9mol£¨∆‰5%”ÎBaCl2»Ð“∫∑¥”¶£¨…˙≥…¡ÚÀ·±µ0.045mol£¨÷ ¡ø «10.485g£¨±£¡Ù“ªŒª–° ˝ «10.5g°£

¡∑œ∞≤·œµ¡–¥∞∏
 «·À…∂·π⁄»´ƒÐ’∆øÿæÌœµ¡–¥∞∏
«·À…∂·π⁄»´ƒÐ’∆øÿæÌœµ¡–¥∞∏
œýπÿƒø
 N2(g)£´CO2(g)£´2H2O(g) ¶§H£Ωa kJ/mol ‘⁄Œ¬∂»T1∫ÕT2 ±£¨∑÷±Ω´0£Æ50 mol CH4∫Õ1£Æ2 mol NO2≥‰»Îê˝Œ™1 Lµƒ√б’»ð∆˜÷–£¨≤‚µ√n(CH4)ÀÊ ±º‰±‰ªØ ˝æð»Áœ¬±Ì£∫
N2(g)£´CO2(g)£´2H2O(g) ¶§H£Ωa kJ/mol ‘⁄Œ¬∂»T1∫ÕT2 ±£¨∑÷±Ω´0£Æ50 mol CH4∫Õ1£Æ2 mol NO2≥‰»Îê˝Œ™1 Lµƒ√б’»ð∆˜÷–£¨≤‚µ√n(CH4)ÀÊ ±º‰±‰ªØ ˝æð»Áœ¬±Ì£∫ 2C(g)+D(g) °˜H<0µƒ∆Ω∫‚“∆∂ØÕºœÒ£¨”∞œÏ∆Ω∫‚“∆∂صƒ‘≠“Úø…ƒÐ « ( )
2C(g)+D(g) °˜H<0µƒ∆Ω∫‚“∆∂ØÕºœÒ£¨”∞œÏ∆Ω∫‚“∆∂صƒ‘≠“Úø…ƒÐ « ( )
 CO(g)£´H2(g)
CO(g)£´H2(g)  CO(g)£´H2O(g) ∑¥”¶π˝≥Ã÷–≤‚∂®µƒ≤ø∑÷ ˝æðº˚œ¬±Ì£®±Ì÷–t2£æt1£©:
CO(g)£´H2O(g) ∑¥”¶π˝≥Ã÷–≤‚∂®µƒ≤ø∑÷ ˝æðº˚œ¬±Ì£®±Ì÷–t2£æt1£©: CH3OCH3(g) + H2O(g)°£“—÷™“ª∂®Ãıº˛œ¬£¨∏√∑¥”¶÷–COµƒ∆Ω∫‚◊™ªØ¬ ÀÊŒ¬∂»°¢Õ∂¡œ±»[n(H2) / n(CO)]µƒ±‰ªØ«˙œþ»Áœ¬◊ÛÕº£∫
CH3OCH3(g) + H2O(g)°£“—÷™“ª∂®Ãıº˛œ¬£¨∏√∑¥”¶÷–COµƒ∆Ω∫‚◊™ªØ¬ ÀÊŒ¬∂»°¢Õ∂¡œ±»[n(H2) / n(CO)]µƒ±‰ªØ«˙œþ»Áœ¬◊ÛÕº£∫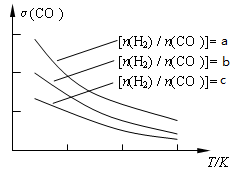
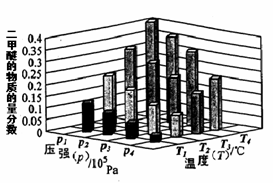
 O2NC6H4COO-+C2H5OH.¡Ω÷÷∑¥”¶ŒÔµƒ≥ı º≈®∂»æ˘Œ™0.050mol/L,15 °Ê ±≤‚µ√£∫O2NC6H4COOC2H5µƒ◊™ªØ¬ ¶¡ÀÊ ±º‰±‰ªØµƒ ˝æð»Á±ÌÀ˘ æ°£ªÿ¥œ¬¡–Œ £∫
O2NC6H4COO-+C2H5OH.¡Ω÷÷∑¥”¶ŒÔµƒ≥ı º≈®∂»æ˘Œ™0.050mol/L,15 °Ê ±≤‚µ√£∫O2NC6H4COOC2H5µƒ◊™ªØ¬ ¶¡ÀÊ ±º‰±‰ªØµƒ ˝æð»Á±ÌÀ˘ æ°£ªÿ¥œ¬¡–Œ £∫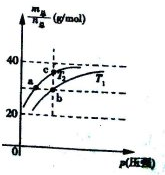
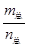 =69g/mol ±£¨»Ùn(NO2)£∫n(N2O4)=2£∫1£¨
=69g/mol ±£¨»Ùn(NO2)£∫n(N2O4)=2£∫1£¨