��Ŀ����
6��NiSO4•6H2O��һ����ɫ������ˮ�ľ��壬�㷺���ڻ�ѧ������������صȣ����Ե�Ʒ������������⣬������Cu��Zn��Fe��Cr�����ʣ�Ϊԭ�ϻ�ã�����������ͼ1��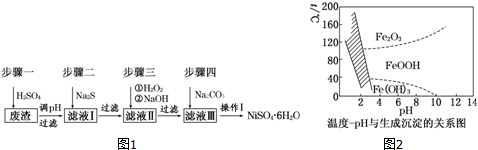
��ش��������⣺
��1����ϡ�����ܽ����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�м��Ȼ�������������Ũ�ȵȣ���дһ�㣩��
��2������Һ�е���������Na2S��Һ��Ŀ���dz�ȥCu2+��Zn2+��д����ȥCu2+�����ӷ���ʽ��Cu2++S2-�TCuS����
��3����40�����ң���6%��H2O2����Fe2+������95��ʱ����NaOH����pH����ȥ���������⣬������NaClO3�����������ڽ�С��pH������ˮ�⣬��������һ��dz��ɫ�Ļ�������[Na2Fe6��SO4��4��OH��12]������ȥ����ͼ2���¶�pH�����ɵij�����ϵͼ��ͼ����Ӱ�����ǻ������ȶ����ڵ�����[��֪25��ʱ��Fe��OH��3��Ksp=2.64��10-39]������˵����ȷ����cd��ѡ����ţ���
a��FeOOH����Ϊ+2��
b������25��ʱ����H2O2����Fe2+������pH=4ʱ��ȥ������ʱ��Һ��c��Fe3+��=2.64��10-29 mol/L
c��������������������������Fe2+�����ӷ���ʽΪ6Fe2++ClO3-+6H+�T6Fe3++Cl-+3H2O
d����ҵ�����г�������85��95�����ɻ������ƣ���ʱˮ���pHΪ1.2��1.8
��4��������������Һ�����Ҫ�ɷ���NiSO4��
��5��ȷ����������Na2CO3��Һ������̼��������ȫ�����ļ�ʵ�鷽�����ϲ���Һ����ɫ��
��6���������ʵ�鲽������Ϊ��ʵ���п�ѡ�õ��Լ���6mol•L-1��H2SO4��Һ������ˮ��pH��ֽ����
�ٹ��ˣ���������ˮϴ��������
��������м�6 mol•L-1��H2SO4��Һ��ֱ��ǡ����ȫ�ܽ⣻
������Ũ������ȴ�ᾧ�����˵�NiSO4•6H2O���壻
���������Ҵ�ϴ��NiSO4•6H2O���岢���ɣ�
���� �������������⣬������Cu��Zn��Fe��Cr��Ԫ�صĻ��������ʣ����������ܽ����pH����˺��ȥ�����ӣ���Һ���ж��������ӡ����۸����ӡ�ͭ���ӡ�п���ӵ����ʣ���ҺI�м��������γ�CuS��ZnS�������ɳ�ȥͭ���ӡ�п���ӣ����ˣ���ҺII�м�H2O2�ǽ�����������������������ͨ������pHʹ�����������۸�������������ij�������ȥ����Һ���п����������Σ���Ҫ��NiSO4������Na2SO4���ټ�̼���Ƴ��������������ˡ�ϴ�ӣ�Ȼ�������ᷴӦ����NiSO4���壻
��1����������Һ��Ļ��������¶ȡ�����Ũ�ȵȣ��ɼӿ췴Ӧ���ʣ�
��2����Na2S��������CuS������
��3��a�����ݻ��ϼ۵Ĵ�����Ϊ�������
b��pH=4��c��OH-��=1��10-10 mol•L-1������Ksp���������ӵ�Ũ�ȣ�
c��������������������������Fe2+���������Ӻ������ӣ�
d������ͼ���ж����ɻ������Ƶ�������
��4���������̷����жϣ�
��5������Ni2+����Һ����ɫ��
��6������NiSO4��Һ�м�̼���ƣ��õ�NiCO3���������ˡ�ϴ�ӣ�������м������ܽ⣬�õ�NiSO4��Һ���پ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵȲ�����NiSO4•6H2O���壮
��� �⣺�������������⣬������Cu��Zn��Fe��Cr��Ԫ�صĻ��������ʣ����������ܽ����pH����˺��ȥ�����ӣ���Һ���ж��������ӡ����۸����ӡ�ͭ���ӡ�п���ӵ����ʣ���ҺI�м��������γ�CuS��ZnS�������ɳ�ȥͭ���ӡ�п���ӣ����ˣ���ҺII�м�H2O2�ǽ�����������������������ͨ������pHʹ�����������۸�������������ij�������ȥ����Һ���п����������Σ���Ҫ��NiSO4������Na2SO4���ټ�̼���Ƴ��������������ˡ�ϴ�ӣ�Ȼ�������ᷴӦ����NiSO4���壻
��1����������Һ��Ļ��������¶ȡ�����Ũ�ȵȣ��ɼӿ췴Ӧ���ʣ�����Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�м��Ȼ�������������Ũ�ȵȣ�
�ʴ�Ϊ�����Ȼ�������������Ũ�ȵȣ�
��2����Na2S��������CuS�������䷴Ӧ�����ӷ���ʽΪ��Cu2++S2-�TCuS����
�ʴ�Ϊ��Cu2++S2-�TCuS����
��3��a��FeOOH��OΪ-2�ۣ�HΪ+1�ۣ�����Ϊ+3�ۣ���a����
b��pH=4��c��OH-��=1��10-10 mol•L-1��Ksp=c��Fe3+����c3��OH-������c��Fe3+��=$\frac{Ksp}{{c}^{3}��O{H}^{-}��}$=$\frac{2.64��1{0}^{-39}}{��1{0}^{-10}��^{3}}$=2.64��10-9 mol/L����b����
c��������������������������Fe2+���������Ӻ������ӣ��䷴Ӧ�����ӷ���ʽΪ��6Fe2++ClO3-+6H+�T6Fe3++Cl-+3H2O����c��ȷ��
d����ͼ���жϿ�֪���ɻ������Ƶ�����Ϊ���¶�Ϊ85��95�棬ˮ���pHΪ1.2��1.8����d��ȷ��
�ʴ�Ϊ��cd��
��4�������̷�����֪��Һ�����Ҫ�ɷ���NiSO4��
�ʴ�Ϊ��NiSO4��
��5������Ni2+����Һ����ɫ������Һ����ɫ��Ϊ��ɫ��˵���������Ѿ���ȫ������
�ʴ�Ϊ���ϲ���Һ����ɫ��
��6������NiSO4��Һ�м�̼���ƣ��õ�NiCO3���������ˡ�ϴ�ӣ�������м������ܽ⣬�õ�NiSO4��Һ���پ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵȲ�����NiSO4•6H2O���壬�����Һ����ȡ�������IJ���Ϊ��
�ٹ��ˣ���������ˮϴ��������
��������м�6 mol•L-1��H2SO4��Һ��ֱ��ǡ����ȫ�ܽ⣻
������Ũ������ȴ�ᾧ�����˵�NiSO4•6H2O���壻
���������Ҵ�ϴ��NiSO4•6H2O���岢���ɣ�
�ʴ�Ϊ�����ˣ���������ˮϴ��������������м�6 mol•L-1��H2SO4��Һ��ֱ��ǡ����ȫ�ܽ⣮
���� ���⿼�����ʵķ����ᴿ��ʵ�鷽������ƣ�Ϊ�߿��������ͣ�������ѧ���ķ���������ʵ�������Ŀ��飬��Ŀ�ѶȽϴ���ȷʵ���Ŀ�ĺ�ԭ���ǽ�����Ĺؼ���ע����ջ���ʵ�������

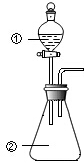 ��ͼ��ʾװ�ý�������ʵ�飺��������Һ������У�Ԥ���������ʵ��������ǣ�������
��ͼ��ʾװ�ý�������ʵ�飺��������Һ������У�Ԥ���������ʵ��������ǣ������� | ѡ�� | �������� | �������� | Ԥ����е����� |
| A | ϡ���� | ̼�������������ƵĻ����Һ | ������������ |
| B | Ũ���� | ��ɰֽ��ĥ�������� | ��������ɫ���� |
| C | �Ȼ�����Һ | Ũ����������Һ | ����������ɫ���� |
| D | ������Һ | �������������Һ | ��Һ����ɫ |
| A�� | A | B�� | B | C�� | C | D�� | D |
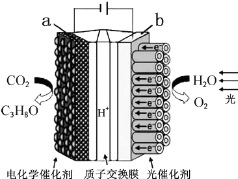
| A�� | ��װ�ý���ѧ��ת��Ϊ���ܺ͵��� | |
| B�� | ��װ�ù���ʱ��H+��b������a����Ǩ�� | |
| C�� | ÿ����1mol O2����44g CO2����ԭ | |
| D�� | a�缫�ķ�ӦΪ��3CO2+18H+-18e-=C3H8O+5H2O |
| ���������� | ���� | |
| A | ��AgCl����Һ�м���NaI��Һʱ���ֻ�ɫ���� | Ksp��AgCl����Ksp��AgI�� |
| B | ��ij��Һ�еμ���ˮ���ټ���KSCN��Һ����Һ�ʺ�ɫ | ��Һ��һ������Fe2+ |
| C | ��NaBr��Һ�е���������ˮ�ͱ��������ã���Һ�ϲ�ʳȺ�ɫ | Br-��ԭ��ǿ��Cl- |
| D | ����ʢ��NH4Cl������Թܣ��Թܵײ�������ʧ���Թܿ��о������� | NH4Cl����������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 2s22p3��2s22p4 | B�� | 3s23p4��2s22p4 | C�� | 3s2��2s22p3 | D�� | 3s23p1��3s23p4 |
| A�� | C3H6��CH2�TCHCH3 | B�� | H2O2�� | C�� |  | D�� |  1S22S22p63S23p6 1S22S22p63S23p6 |
| ѡ�� | ������ | ������ |
| A | SO2��Ư���� | SO2��ʹ��ˮ�����Ը��������Һ��ɫ |
| B | Fe3+��ǿ������ | FeCl3��Һ�����ڻ��շϾɵ�·���е�ͭ |
| C | Ũ��������ˮ�� | Ũ�����ʹ���Ǻ�ֽ��̼����� |
| D | SiO2�е����� | SiO2�������Ʊ����ά |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ������������֬���������࣬������ͬϵ�� | |
| B�� | ����ؽ����Σ��������ö�����ţ�̿ɽⶾ | |
| C�� | ���ǡ���ά�ء�����ˮ������ղ��ﶼ�������� | |
| D�� | ú�ĸ�����Եõ����ȷ����� |
 ��
��