��Ŀ����
11����A��B��C��D��E��F��6�ֶ�����Ԫ�أ�����A ��һ��ԭ�Ӳ������ӣ�B �ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ�����������ӣ�C ԭ�������ĵ����Ų�ʽΪʱnsnnp2n��D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С��E�ĵ�����һ�ֱ����ж������������������壻F Ԫ���������������۵Ĵ�����Ϊ4����1��B��C��E ��Ԫ����ɵĻ�����֮һ���Ǽ�������������Ҫ�ɷ֣������Ļ�ѧ�������Ӽ������ۼ��������ʽΪ
 ��
����2��D��E��F �ļ����Ӱ뾶�ɴ�С��˳����S2-��Cl-��Al3+�������ӷ��ű�ʾ����
��3�����־���A��B��C��F ����Ԫ�صĻ���������Һ�����Ӧ�����ӷ���ʽH++HSO3-=SO2��+H2O��
��4��һ������ʯ������ͨ��һ������E ���ʣ�����ǡ����ȫ��Ӧ���������������ֺ�EԪ�ص����ӣ������������ӵ����ʵ�����n�� �뷴Ӧʱ�䣨t����������ͼ��ʾ��

��t2ʱ������������������Ϊ37g����ʱ��Ӧ�Ļ�ѧ����ʽΪ10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O��
��5��A��B �γɵĻ�����BA ���л��ϳ�����;�ܹ㷺�������Զ�ȡ�ܶ�����е����Ӷ�������Ӧ���ƵĻ����д�������Ҵ���Ӧ�Ļ�ѧ����ʽNaH+CH3CH2OH��CH3CH2ONa+H2����
���� ��A��B��C��D��E��F��6�ֶ�����Ԫ�أ�����A ��һ��ԭ�Ӳ������ӣ���AΪH��B �ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ�����������ӣ���BΪNa��C ԭ�������ĵ����Ų�ʽΪʱnsnnp2n��n=2����֪C��������Ϊ8����CΪO��D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С����DΪAl��E�ĵ�����һ�ֱ����ж������������������壬��֪����Ϊ��������EΪCl��FԪ���������������۵Ĵ�����Ϊ4�����Ϊ+6����ͼ�Ϊ-2����FΪS��
��1��B��C��E ��Ԫ����ɵĻ�����֮һ���Ǽ�������������Ҫ�ɷ�ΪNaClO��
��2�����Ӳ�ṹ��ͬ�˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ��
��3�����־���H��Na��O��S����Ԫ�صĻ���������Һ�������Ӧ��Ӧ���������������������ƣ�
��4����ͼ���֪֪������������ӵ����ʵ���Ϊ0.2mol����������ӵ����ʵ���Ϊ0.1mol�����Դ���������ӵ����ʵ�������������ӵ����ʵ���֮��Ϊ2��1�����ݵ�ʧ�����غ�֪�������������Ƶķ�Ӧ����ʽ���Դ˼��㣻
��5��NaH�����Ҵ�����ȡ����Ӧ��
��� �⣺��A��B��C��D��E��F��6�ֶ�����Ԫ�أ�����A ��һ��ԭ�Ӳ������ӣ���AΪH��B �ĵ���������ˮ���ҷ�Ӧ������ǿ������Һ�к������ֵ�������ͬ�����������ӣ���BΪNa��C ԭ�������ĵ����Ų�ʽΪʱnsnnp2n��n=2����֪C��������Ϊ8����CΪO��D��Bͬ���ڣ���������D�ļ����Ӱ뾶��С����DΪAl��E�ĵ�����һ�ֱ����ж������������������壬��֪����Ϊ��������EΪCl��FԪ���������������۵Ĵ�����Ϊ4�����Ϊ+6����ͼ�Ϊ-2����FΪS��
��1��B��C��E ��Ԫ����ɵĻ�����֮һ���Ǽ�������������Ҫ�ɷ�ΪNaClO�������Ӽ������ۼ��������ʽΪ ��
��
�ʴ�Ϊ�����Ӽ������ۼ��� ��
��
��2�����Ӳ�ṹ��ͬ�˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶�ɴ�С��˳���ǣ�S2-��Cl-��Al3+��
�ʴ�Ϊ��S2-��Cl-��Al3+��
��3�����־���H��Na��O��S����Ԫ�صĻ���������Һ�������Ӧ��Ӧ���������������������ƣ���Ӧ���ӷ���ʽΪH++HSO3-=SO2��+H2O��
�ʴ�Ϊ��H++HSO3-=SO2��+H2O��
��4����ͼ���֪֪������������ӵ����ʵ���Ϊ0.2mol����������ӵ����ʵ���Ϊ0.1mol�����Դ���������ӵ����ʵ�������������ӵ����ʵ���֮��Ϊ2��1�����ݵ�ʧ�����غ�֪���������������Ƶķ�Ӧ����ʽΪ10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O��n��Cl��=2n��CaCl2��+2n[Ca��ClO��2]+2n[Ca��ClO��3]=0.07mol+0.2mol+0.1mol=1mol����n��Ca��=0.5mol����t2ʱ������������������Ϊ0.5mol��74g/mol=37g��
�ʴ�Ϊ��37��10Cl2+10Ca��OH��2=7CaCl2+2Ca��ClO��2+Ca��ClO3��2+10H2O��
��5��H��Na�γɵĻ�����NaHBA���л��ϳ�����;�ܹ㷺�������Զ�ȡ�ܶ�����е����Ӷ�������Ӧ���ƵĻ���������Ҵ���Ӧ�Ļ�ѧ����ʽΪ��NaH+CH3CH2OH��CH3CH2ONa+H2����
�ʴ�Ϊ��NaH+CH3CH2OH��CH3CH2ONa+H2����
���� ���⿼��λ�ýṹ�����ʼ�Ԫ�����ڱ���Ԫ�������ɣ�Ϊ��Ƶ���㣬����Ԫ�ص��ƶ�Ϊ���Ĺؼ����漰���ӷ�Ӧ��������ԭ��Ӧ���л�������ʵȣ��ۺ��Խ�ǿ��ע�ػ���֪ʶ�Ŀ��飬��Ŀ�ѶȲ���

| A�� | ���ʵķе㣺W��X | B�� | �����ӵĻ�ԭ�ԣ�W��Z | ||
| C�� | �������ˮ��������ԣ�Y��Z | D�� | X��Y���ܴ�����ͬһ���ӻ������� |
| X | ||
| Y | Z | W |
| T |
| A�� | X��W��ZԪ�ص�ԭ�Ӱ뾶�����ǵ���̬�⻯������ȶ��Ծ����ε��� | |
| B�� | Y��Z��WԪ������Ȼ���о�����������̬���ڣ����ǵ�����������ˮ������������ε��� | |
| C�� | YX2�����ۻ���Һ̬WX3��������˷����Ӽ������� | |
| D�� | ����Ԫ�������ɣ������Ʋ�TԪ�صĵ��ʾ��а뵼�����ԣ�T2X3���������Ժͻ�ԭ�� |
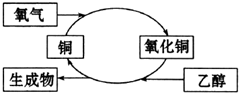
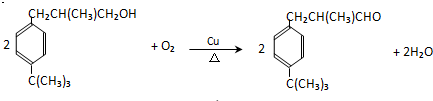
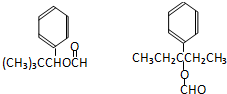
| A�� | ����������ȩ | B�� | �Ҵ������˻�ԭ��Ӧ | ||
| C�� | ͭ�Ǵ˷�Ӧ�Ĵ��� | D�� | ��Ӧ���к����ɫ����仯������ |
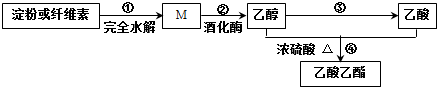
����˵����ȷ���ǣ�������
| A�� | ���ۺ���ά�ػ�Ϊͬ���칹�� | |
| B�� | ��Ӧ�ڣ�1 mol M������3 mol CH3CH2OH | |
| C�� | ��Ӧ�ۣ����跴Ӧ������Ϊ�����ظ������Һ | |
| D�� | ��Ӧ�ܣ�����ͨ����з�̪�ı���̼������Һ�������ú��²���Һ��ɫ�ޱ仯 |

 ����ƽ���ͷ���
����ƽ���ͷ���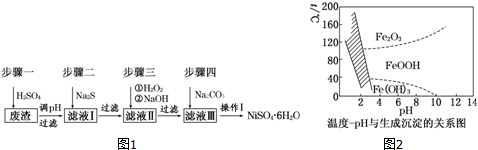
 ��D�ĺ˴Ź�������ͼ�н�����4�ַ壻
��D�ĺ˴Ź�������ͼ�н�����4�ַ壻 ��
��
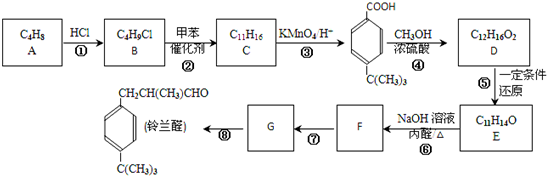
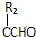
 $\stackrel{����}{��}$
$\stackrel{����}{��}$ +HCl��R��R1��R2��������
+HCl��R��R1��R2��������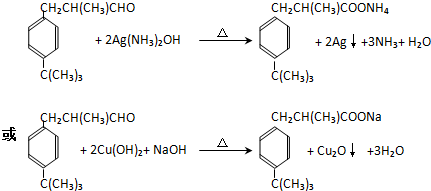 ��
�� ��
�� ��
�� ��
��