��Ŀ����
11��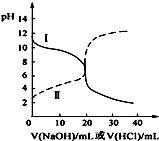 25��ʱ��ȡŨ�Ⱦ�Ϊ0.1mol•L-1�Ĵ�����Һ�Ͱ�ˮ��Һ��20mL���ֱ���0.1mol•L-1NaOH��Һ��0.1mol•L-1��������к͵ζ����ζ�������pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
25��ʱ��ȡŨ�Ⱦ�Ϊ0.1mol•L-1�Ĵ�����Һ�Ͱ�ˮ��Һ��20mL���ֱ���0.1mol•L-1NaOH��Һ��0.1mol•L-1��������к͵ζ����ζ�������pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ���ߢμ���Һ��10mLʱ��c��CH3COO-����c��Na+����c��H+����c��OH-�� | |
| B�� | ���ߢμ���Һ��10 mLʱ��c��CH3COO-��-c��CH3COOH��=2[c��OH-��-c��H+��] | |
| C�� | ���ߢμ���Һ��10 mL��20 mL֮����ڣ�c��NH4+��=c��Cl-����c��OH-��=c��H+�� | |
| D�� | ���ߢμ���Һ��20 mLʱ��c��Cl-����c��NH4+����c��H+����c��NH3•H2O����c��OH-�� |
���� �������߱仯����֪����δ�μ���Һʱ������I��pH��7��˵��������ζ�����Һ���������߱仯����֪����δ�μ���Һʱ������II��pH��7��˵�����ڼ�ζ�����Һ��
A������IΪ��ζ�����Һ�����μ���Һ��10mLʱ����Һ�е��������Ȼ�狀�һˮ�ϰ���
B������IIΪ�������Ƶζ�������Һ�����ߣ��μ���Һ��10 mLʱ����Һ�е������ǵ����ʵ���Ũ�ȵĴ���ʹ����ƣ���������غ㼰����غ������
C������IIΪ�������Ƶζ�������Һ�����ߣ��μ���Һ��10 mL��20 mL֮�䣬��Һ�е������Ǵ����ƺʹ��ᣬ�������̶ȴ��ڴ��������ˮ��̶ȣ�
D����IΪ��ζ�����Һ�����μ���Һ��20mLʱ����Һ�е��������Ȼ�泥�笠�����ˮ�⣬��ˮ��̶Ⱥ�����
��� �⣺�������߱仯����֪����δ�μ���Һʱ������I��pH��7��˵��������ζ�����Һ���������߱仯����֪����δ�μ���Һʱ������II��pH��7��˵�����ڼ�ζ�����Һ��
A������IΪ��ζ�����Һ�����μ���Һ��10mLʱ����Һ�е��������Ȼ�狀�һˮ�ϰ�����Һ�ʼ��ԣ���c��H+����c��OH-������A����
B������IIΪ�������Ƶζ�������Һ�����ߣ��μ���Һ��10 mLʱ����Һ�е������ǵ����ʵ���Ũ�ȵĴ���ʹ����ƣ���Һ�д��������غ�c��CH3COO-��+c��CH3COOH��=2c��Na+������Һ�д��ڵ���غ�c��CH3COO-��+c��OH-��=c��H+��+c��Na+�������Ե�c��CH3COO-��-c��CH3COOH��=2[c��H+��-c��OH-��]����B����
C������IIΪ�������Ƶζ�������Һ�����ߣ��μ���Һ��10 mL��20 mL֮�䣬��Һ�е������Ǵ����ƺʹ��ᣬ�������̶ȴ��ڴ��������ˮ��̶ȣ�����Һ��c��H+����c��OH-������C����
D������IΪ��ζ�����Һ�����μ���Һ��20mLʱ����Һ�е��������Ȼ�泥���Һ�����ԣ���c��H+����c��OH-������Һ�д��ڵ���غ�c��Cl-��+c��OH-��=c��NH4+��+c��H+�������Ե�c��Cl-����c��NH4+��������ˮ��̶Ƚ�С��ˮ����������ӣ���������Ũ�ȴ�С˳����c��Cl-����c��NH4+����c��H+����c��NH3•H2O����c��OH-������D��ȷ��
��ѡD��
���� ���⿼�����������Һ�����жϣ�Ϊ��Ƶ���㣬���յζ����ߵ��жϼ���Һ�е����ʡ�����غ�������غ�Ϊ���Ĺؼ������ط�����Ӧ���������ۺϿ��飬��Ŀ�Ѷ��еȣ�

| A�� | ����ˮ���������� | B�� | ʯ���ѽ��Ʊ�ϩ | ||
| C�� | �Ҵ������ᷴӦ���������� | D�� | ��֬��ŨNaOH��Ӧ�Ƹ�֬������ |
| A�� | ʹ��LED���������ڡ���̼�����ʽ | |
| B�� | ���Ƹ����ܵ���ĥ��̥���ɼ���ϸ�����PM2.5���IJ��� | |
| C�� | �ڼ��õ���ˮ��������ڵ���Ƕþ�����Է�ֹ�ڵ�����ʴ | |
| D�� | ʩ������ʯ����ɽ����μ�أ����϶�NaCl��Na2CO3���ļ��� |
| A�� | pH=1����Һ�У�Fe2+��Cl-��NO3-��K+ | |
| B�� | �����̪�Ժ�ɫ����Һ�У�Na+��Al3+��CO32-��AlO2- | |
| C�� | �������۲���H2����Һ�У�Fe2+��Na+��SO42-��ClO- | |
| D�� | 0.1mol•L-1 NaHCO3��Һ�У�Na+��NH4+��SO42-��NO3- |
| ѡ�� | ʵ���������ʵ | ʵ��Ŀ�Ļ���� |
| A | ����ɫ��Һ$\stackrel{NaOH��Һ}{��}$���ɫ���� | ˵��ԭ��Һ��һ��������FeCl3 |
| B | CaO$\stackrel{H_{2}O}{��}$Ca��OH��2$\stackrel{Na_{2}CO_{3}}{��}$NaOH | ����ʯ���Ʊ�NaOH��Һ |
| C | ���ռ�������$\stackrel{Ba��NO_{3}��_{2}��Һ}{��}$��ɫ���� | ������һ������SO42- |
| D | H3PO3+2NaOH��������-Na2HPO3+2H2O | H3PO3������Ԫ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �ױ��������ڹ����·�Ӧ��Ҫ����2��4-���ȼױ� | |
| B�� | ���Ҵ���Ũ�����Ʊ���ϩʱ������ˮԡ���ȿ��Ʒ�Ӧ���¶� | |
| C�� | 1 mol��������ˮ������2 mol CH3CH2OH��2 mol CO2 | |
| D�� | ʵ�������ᴿ��������������Ҵ����ɲ����ȼ���ʯ�ң����˺�������ķ��� |
 �Ѷ�����һ����Ҫ���л���Ԫ�ᣬ��Ҫ������������66��ά������66��֬����ʵ�����п�ͨ����������������Ӧ���Ʊ�����Ӧԭ����3
�Ѷ�����һ����Ҫ���л���Ԫ�ᣬ��Ҫ������������66��ά������66��֬����ʵ�����п�ͨ����������������Ӧ���Ʊ�����Ӧԭ����3 +8HNO3$\stackrel{һ������}{��}$3HOOC��CH2��4COOH+8NO��+7H2O����Ӧװ�ã����ּг�װ�ú���Դ��ʡ�ԣ���ͼ��ʾ��
+8HNO3$\stackrel{һ������}{��}$3HOOC��CH2��4COOH+8NO��+7H2O����Ӧװ�ã����ּг�װ�ú���Դ��ʡ�ԣ���ͼ��ʾ�� ��1�����ʯ��ʯī�ľ�����ͼ����̼ԭ�Ӱ뾶Ϊa cm �����������̼ԭ�����У������ӵ�����ΪNA������ʯ���ܶȱ���ʽΪ��Ҫ������Ϊ��ĸ�������ŵı���ʽ��$\frac{9\sqrt{3}}{1{6a}^{3}{N}_{A}}$g/cm3��һ��ʯī�����ں���4��̼ԭ�ӣ�ʯī���۵�Ƚ��ʯ���۵�Ҫ�ߣ���ԭ����ʯī��̼̼����С�ڽ��ʯ��̼̼�����������־�����̼����λ����ÿ��̼��Χ��֮�����̼ԭ������֮��Ϊ4��3��
��1�����ʯ��ʯī�ľ�����ͼ����̼ԭ�Ӱ뾶Ϊa cm �����������̼ԭ�����У������ӵ�����ΪNA������ʯ���ܶȱ���ʽΪ��Ҫ������Ϊ��ĸ�������ŵı���ʽ��$\frac{9\sqrt{3}}{1{6a}^{3}{N}_{A}}$g/cm3��һ��ʯī�����ں���4��̼ԭ�ӣ�ʯī���۵�Ƚ��ʯ���۵�Ҫ�ߣ���ԭ����ʯī��̼̼����С�ڽ��ʯ��̼̼�����������־�����̼����λ����ÿ��̼��Χ��֮�����̼ԭ������֮��Ϊ4��3��
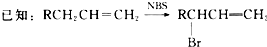
 ��
�� ��
��