��Ŀ����
1����֪��2Na2O2+2H2O��4NaOH+O2��ij��ѧС���ͬѧ����˰��Ĵ�����ʵ�飬װ����ͼ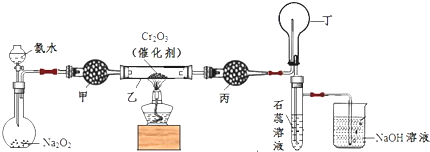
��1����Һ©���а�ˮ��Ũ��Ϊ9.0mol/L��������������Ϊ0.35�ܶ�Ϊ0.88g/cm3�İ�ˮ����9��.0mol/L�İ�ˮ100mL����Ҫ����������a��c��ѡ���ţ�
a.100mL ����ƿ b.10mL��Ͳc.50mL ��Ͳ d��������ƽ
��2������ʱ�����з�Ӧ�Ļ�ѧ����ʽΪ
 ���ڸ÷���ʽ�б�������ת�Ƶķ������Ŀ
���ڸ÷���ʽ�б�������ת�Ƶķ������Ŀ��3��ʵ�鿪ʼ�ȼ��ȴ����������������ʱ�ٴ�Һ©�����������߾ƾ��ƣ��ɹ۲쵽�������У�
��ƽ����ƿ�в��ϲ������壬�������ֺ���
�ڶ�����ƿ����������ɫת��Ϊ����ɫ
���Թ����ʯ����Һ����ɫ��Ϊ��ɫ
��4������ܼ���ʢ�ż�ʯ�ң�������ˮ������ܱ��������ռ������壬����ʢ�ŵ��Լ������ǣ�ѡ�����б�ţ�b
a��Ũ���� b����̬P2O5 c����ʯ�ң�
���� ��1������C=$\frac{1000�Ѧ�}{M}$����Ũ��ˮ�����ʵ���Ũ�ȣ��ٸ���Ũ��ˮϡ��ǰ�����ʵ����ʵ���������㣻
��2������ʱ������Ϊ�����������ڴ������������·�����Ӧ����һ��������ˮ����Ӧ��NH3��4NO��NԪ�صĻ��ϼ���-3������Ϊ+2�ۣ�OԪ�ػ��ϼ���0�۽��͵�-2�ۣ�
��3��ʵ�鿪ʼ�ȼ��ȴ����������������ʱ�ٴ�Һ©�����������߾ƾ��ƣ������ɵ�һ��������װ�õ�������Ӧ���ɺ���ɫ�Ķ����������壬������������ʯ����Һ�������������ԣ�����ʯ����Һ����ɫ��Ϊ��ɫ���ݴ˷�����
��4������ܼ������Ǹ��������Ͱ����Ļ�����壻��Ϊ�������ܣ�Ϊ�˳�ȥ�����İ�����ˮ����������ʢ�ŵ�ҩƷΪ����ҩƷ��̬P2O5��
��� �⣺��1��Ũ��ˮ��Ũ��C=$\frac{1000�Ѧ�}{M}$=$\frac{1000��0.88g/c{m}^{3}��35%}{17g/mol}$=18.1mol/L��Ũ��ˮϡ��ǰ�����ʵ����ʵ������䣬��Ũ��ˮ�����ΪV������18.1mol/L��V=9.0mol/L��0.1L��V=0.0497L=49.7mL������ѡ��100mL����ƿ��50mL��Ͳ��
��ѡ��a��c��
��2������ʱ������Ϊ�����������ڴ������������·�����Ӧ����һ��������ˮ������ʽΪ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O����Ӧ��OԪ�صõ��ӣ����ϼ۽��ͣ�NԪ��ʧ���ӣ����ϼ����ߣ�ת�Ƶ�����ĿΪ20������ת�Ƶķ������Ŀ�ɱ�ʾΪ ��
��
�ʴ�Ϊ�� ��
��
��3��ʵ�鿪ʼ�ȼ��ȴ����������������ʱ�ٴ�Һ©�����������߾ƾ��ƣ������ɵ�һ��������װ�õ�������Ӧ���ɺ���ɫ�Ķ����������壬������������ʯ����Һ�������������ԣ�����ʯ����Һ����ɫ��Ϊ��ɫ�����Կɹ۲쵽������������Cr2O3���ܱ��ֺ���״̬��������ƿ����������ɫת��Ϊ����ɫ���Թ����ʯ����Һ����ɫ��Ϊ��ɫ��
�ʴ�Ϊ��������ƿ����������ɫת��Ϊ����ɫ���Թ����ʯ����Һ����ɫ��Ϊ��ɫ��
��4������ܼ������Ǹ��������Ͱ����Ļ�����壻��Ϊ�������ܣ�Ϊ�˳�ȥ�����İ�����ˮ����������ܱ�Ӧʢ�����ԵĹ�������������ʢ�ŵ�ҩƷΪ��ˮ��̬P2O5��
�ʴ�Ϊ��b��
���� ���⿼�鰱���Ĵ���������Ŀ�Ѷ��еȣ��漰��Һ�����ƣ������Ʊ������������ʣ���������֤��β�����������ճ���������Ʊ�ԭ���ͳ����ǽ���ؼ��������ֿ�����ѧ���ķ�����������������ѧʵ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | F-�Ľṹʾ��ͼ��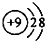 | B�� | ��ϩ���ӱ���ģ�ͣ� | ||
| C�� | ����Ľṹʽ��C2H4O2 | D�� | Na2O�ĵ���ʽ��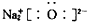 |
| A�� | H2O��D2O��ͬϵ�� | |
| B�� | 35Cl��37Cl��ͬ�������� | |
| C�� | O2��O3��ͬλ�� | |
| D�� | �Ҵ�������ѣ�CH3-O-CH3����ͬ���칹�� |
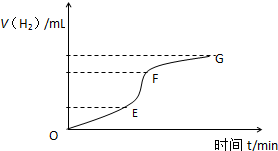 �ô�����п����ϡ���ᷴӦ��ȡ��������ش�
�ô�����п����ϡ���ᷴӦ��ȡ��������ش���1����ͼΪ�����뷴Ӧʱ���ϵͼ�������ж�EF�λ�ѧ��Ӧ������죬����ԭ���Ǹ÷�Ӧ�Ƿ��ȷ�Ӧ��������Һ�Ľ��У���Һ�¶����ߣ���ҺŨ����Ȼ��С�����¶�Ӱ�����Ũ��Ӱ�죬���Է�Ӧ���ʿ죮
��2��ijѧ����100mLϡ�����м���������п�ۣ�����ˮ�������ռ���Ӧ�ų���������ʵ���¼���£��ۼ�ֵ�����������ת��Ϊ��״�����������
| ʱ�䣨min�� | 1 | 2 | 3 | 4 | 5 |
| ���������mL�� | 50 | 120 | 232 | 290 | 310 |
��3��Ϊ�˼���������Ӧ�����ʣ�������Һ�м����������ʣ�����Ϊ���е���AC��
A������ˮ B���Ȼ��ƹ��� C���Ȼ�����Һ D��Ũ����
��4���������������⣬����Ϊ�����Բ�ȡ��Щ��ʩ��������ѧ��Ӧ���ʣ������ٻش�һ�֣������¶ȣ�
| A�� | ̼������Һ | B�� | ʪ�����ɫʯ����ֽ | ||
| C�� | ʪ��ĵ��۵⻯����ֽ | D�� | ��������Һ |
| A�� | �÷�Ӧ������Cl2��ǿ������ | B�� | ���ܵ�©�������ͻ�������� | ||
| C�� | �÷�Ӧ���ڸ��ֽⷴӦ | D�� | ����1molN2��6mol����ת�� |
| A�� | ���MgCl2������Һ���Ƶý���þ | |
| B�� | ��Ũ����������Ӧ����������H2������ | |
| C�� | ��ⱥ��ʳ��ˮ�Ĺ����У�ˮ�ĵ���ƽ�������ƶ� | |
| D�� | �������ڿ�����¶���������绯ѧ��ʴ�����䰵 |
