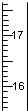
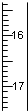
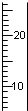
��Ŀ����
1������1mol��������Һ����μ���Ba��OH��2��Һ����ַ�Ӧ������˵������ȷ���ǣ�������| A�� | ��Al3+ǡ����ȫ����ʱ������Ba��OH��2 1.5 mol | |
| B�� | ��SO42-ǡ����ȫ����ʱ��Al3+ȫ��ת��ΪAl��OH��3 | |
| C�� | ������Һ�м���1.5 mol Ba��OH��2ʱ����Ӧ�����������ӷ���ʽ��ʾ��2Al3++3SO42-+3Ba2++6OH-�T2Al��OH��3��+3BaSO4�� | |
| D�� | ���ż����Ba��OH��2�����ʵ������������������ʵ����������� |
���� ��Al3+ǡ��ȫ������ʱ����Ҫ1.5molBa��OH��2��������1.5molBa2+��3molOH-����Ӧ�����ӷ���ʽΪ2Al3++3SO42-+3Ba2++6OH-=3BaSO4��+2Al��OH��3����
�����μ�0.5molBa��OH��2������SO42-+Ba2++2Al��OH��3+2OH-=BaSO4��+2AlO2-+2H2O��ͼ��Ϊ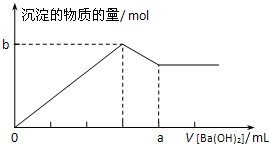 ���Դ˽��
���Դ˽��
��� �⣺��Al3+ǡ��ȫ������ʱ����Ҫ1.5molBa��OH��2��������1.5molBa2+��3molOH-����Ӧ�����ӷ���ʽΪ2Al3++3SO42-+3Ba2++6OH-=3BaSO4��+2Al��OH��3����
�����μ�0.5molBa��OH��2������SO42-+Ba2++2Al��OH��3+2OH-=BaSO4��+2AlO2-+2H2O��ͼ��Ϊ ��
��
A���ӿ�ʼ����Ba��OH��2��1.5molʱ����Ӧ��2KAl��SO4��2+3Ba��OH��2=K2SO4+2Al��OH��3��+3BaSO4�����У�����1molAl3+ǡ����ȫ����ʱ��������������1.5mol����A��ȷ��
B.1mol KAl��SO4��2����Һ�к���2mol��SO42-����SO42-ǡ����ȫ����ʱ����Ҫ2mol��Ba2+����������2mol��Ba��OH��2����KAl��SO4��2+2Ba��OH��2=KAlO2+2BaSO4��+2H2O��Al3+ȫ��ת��Ϊƫ��������ӣ���B����
C��������Һ�м���1.5mol��������ʱ��������Ӧ��2KAl��SO4��2+3Ba��OH��2=K2SO4+2Al��OH��3��+3BaSO4�������ӷ���ʽ��ʾΪ��2Al3++3SO42-+3Ba2++6OH-=2Al��OH��3��+3BaSO4������C��ȷ��
D���������������������ʵ�����������ʼ���������࣬��������������ܽ⣬�ʳ��������ʵ�����������С����D����
��ѡBD��
���� ���⿼�����ӷ���ʽ����д����㣬������ѧ���ķ��������������Ŀ��飬��Ŀ�Ѷ��еȣ�����Ϊ�����йص����ӷ���ʽ����дʱע����ȷ�жϸ����ӷ�Ӧ�ij̶�Ϊ������Ĺؼ���

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�| A�� | ˮ�����߲� | B�� | ��������� | C�� | ������Ѽ�� | D�� | ������ |
| A�� | ����ϡ���ᷴӦ��Fe+2H+=H2��+Fe2+ | |
| B�� | NaHCO3��Һ��NaOH��Һ��Ӧ��OH-+HCO3-=CO32-+H2O | |
| C�� | �Ȼ�����Һ��������ˮ��Ӧ��Al3++4OH-=AlO2-+2H2O | |
| D�� | ����ͭ��Һ������������Һ��Ӧ��Cu2++2OH-=Cu��OH��2�� |
| A�� | ����������Һ�����ᷴӦ��OH-+H+=H2O | |
| B�� | �� FeCl2��Һ��ͨ��Cl2��Fe2++Cl2=Fe3++2Cl- | |
| C�� | С�մ���Һ���ռ���Һ��Ӧ��HCO3-+OH-=CO32-+H2O | |
| D�� | ����ͨ����ˮ�У�Cl2+H2O=Cl-+ClO-+2H+ |
| A�� | ��������ƽ����8.75gʳ�� | |
| B�� | ��500ml������ƿ����480ml��Һ | |
| C�� | ��10ml��Ͳ��ȡ6.46ml���� | |
| D�� | �ù㷺pH��ֽ���ij��Һ��pHΪ4.5 |
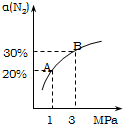 ij�¶��£����ڷ�ӦN2��g��+3H2��g��?2NH3��g������H=-92.4kJ/mol��N2��ƽ��ת���ʣ���������ϵ��ѹǿ��P���Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
ij�¶��£����ڷ�ӦN2��g��+3H2��g��?2NH3��g������H=-92.4kJ/mol��N2��ƽ��ת���ʣ���������ϵ��ѹǿ��P���Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��1.0mol������3.0mol����������1L�ܱ������з�����Ӧ���ų�������Ϊ92.4kJ | |
| B�� | ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A����K��B�� | |
| C�� | ������Ӧ�ڴﵽƽ�������ѹǿ��H2��ת������� | |
| D�� | ����ѹǿ���䣬ͨ��������壬ƽ�ⳣ�����䣬ƽ�ⲻ�ƶ� |
| A�� | ú�� | B�� | ˮ | C�� | ���� | D�� | ���Ȼ�̼ |
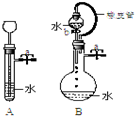 ������ͼ�����ش��������⣺
������ͼ�����ش��������⣺